Principles of Biochemistry 3 |Amino acid to Protein| Class Notes |HarvardX
Primary: Amino acid
 |
|---|
| © HarvardX |
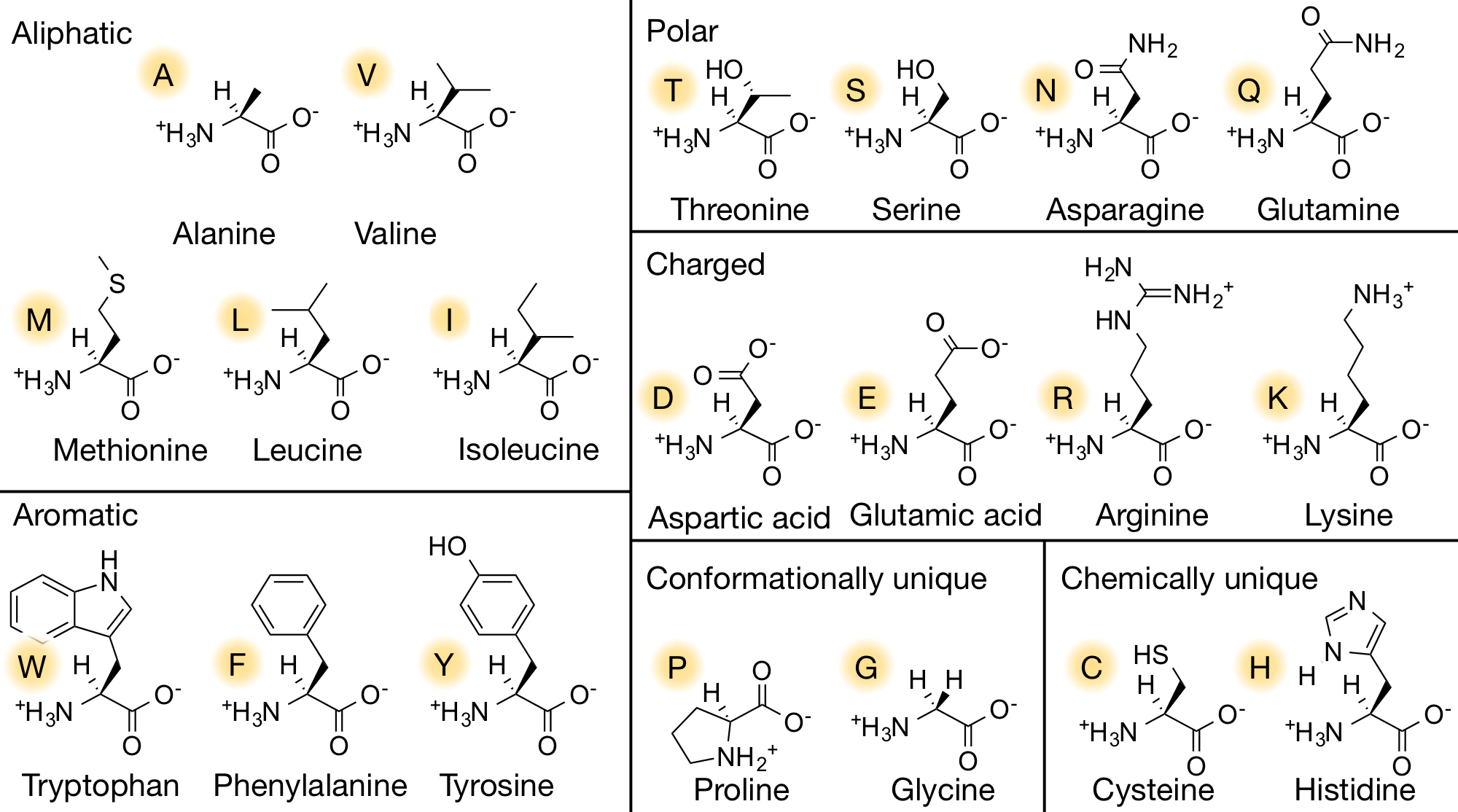
- 4 groups: H group | amino group | carboxyl group | side chain (variable group).
- In the pH of 7.4:
- The amino group is positively charged,
- The carboxyl group is negatively charged.
- As a result, the amino acid is a neutral, zwitterion.
- Chiral, the amino acid made by organisms are all L-amino acids.
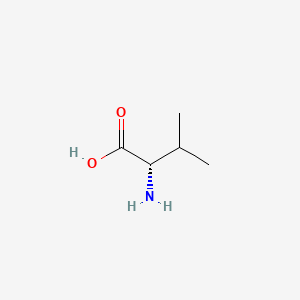
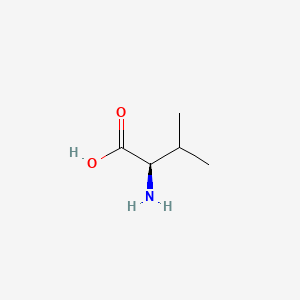
- As you can see in the 2D and 3D models below, when we write the formulae of the L and D form of the valine, we write it from Carboxyl to R Chain. When the Amino group against us is D-form. When the amino away from us is L-from.
- In the formulae, when the amino group on the left and the carboxyl group on the right, the side-chain out of the plane is L-form, divine into the plane is D-form.
| L-Valine | D-Valine |
|---|---|
 |
 |
Interactions between amino aicd
- Van de Waals Interactions: weakest and only in short distances.
- Hydrogen bond: short distances and polar groups.
- Salt bridges (Ionic bond): Strongest non-covalent bond
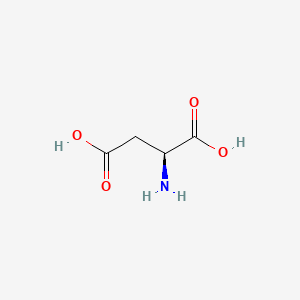
Difference between amino acid
| Aspartate | Glutamate |
|---|---|
 |
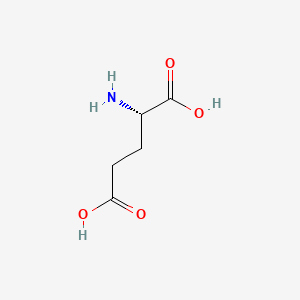 |
Aspartate is similar to Glutamate, which has a longer R chin contributing extra methane. This makes the result different since Glutamate has more number of rotamers. As a result, Glutamate has a better ability to position itself exactly in the right position.
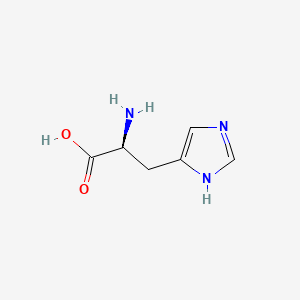
Histidine
Histidine is often found in the activity sites of enzymes.
| Histidine | Imidazole |
|---|---|
 |
 |
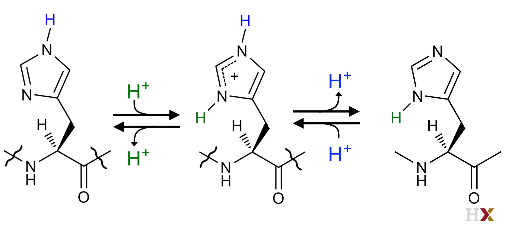
So, it could be either charged or uncharged at the same time in its imidazole group. As a result, it could be an electron shuttle. (From blue H+ to Green H^+ ^)
 |
|---|
| © HarvardX |
Others
-
Tyr is more acidic than Thr/Ser even though there are all alcohols.
-
Sulfur atom:
Methinoine:
Cysteine: Oxidation/Reduction (Disulfide bond). -
Achiral amino acid:
| Glycine | Proline |
|---|---|
Glycine has hydrogen on its side-chin. Proline’s side-chain covalent to the hydrogen group from C. These make proline has less but a unique conformation among other amino acids and contributes to the protein folding.
Primary -> Secondary -> Tertiary -> Quaternary
Secondary: Beta sheet; Alpha helix, and loops.
Peptide Structure
- C-$\alpha$: Tetrahedral structure
- C-Carboxyl: Trigonal structure (which suggest that the peptide bond is a double bond)
- Distance:
- $C_{amines}=N < C_{Carboxyl} -N < C_{amines}-N$
- $C_{carboxyl} - N = 1.32\mathring{A}$
- $C_{amines} - N = 1.469\mathring{A}$
- partial double bond character
- not rotate freely: remain two structure: cis/trans
- Most AA prefers to stay in a trans structure except proline.
- Rotation (dihedral angles) of the $C_{\alpha}$
- $\phi: C_{\alpha}-N$ (amino)
- Measurement:
- look down at the length of the $N-C_{\alpha}$ from its most terminal end
- measure the angle between the two carbonyl carbons.
(PS: Let’s just say, overlap the N and $C_{\alpha}$, and measure the two carbonyl carbon)
- Measurement:
- $\psi: C_{\alpha}-C$ (carboxyl)
- measure: Similarly, let’s overlap the $C_{\alpha}-C$ atoms, and measure the angle of the two amino atoms.
- $\omega: C_{carboxyl} - N = 0^{\circ}/ 180^{\circ}$ (peptide bond)
- $0^{\circ}$: cis-form
- $180^{\circ}$: trans-form
- $\phi: C_{\alpha}-N$ (amino)
Let’s take look at this simplified 3D model. First, we need to identify the $C_{\alpha}$. It is placed right in the center which connected a carbon (grey in left), nitrogen (Blue in right), and two hydrogens (white in up and down)
- When measuring the $\phi$, which is the angle of $C_{\alpha}-N$ bond. After we overlap the $C_{\alpha}$ and $N$ (from N to C), we could measure the angle by looking at carboxyls. In this case, I believe it is $90^{\circ}$.
- When measuring the $\psi$, which is the angle of $C_{\alpha}-C$ bond.
we overlap the $C_{\alpha}$ and $C$ (from $C_{\alpha}$ to $C$ carboxyl),and we can measure the angle by look at Aminos. Which I believe is around $155^{\circ}$ - PS: the angles are from 0 to 180 from clockwise and -0 to -180 from anti-clockwise.
| $\phi$ | $\psi$ |
|---|---|
Ramachandran Plot:
G.N. Ramachandran
- realized that not all $\phi$ and $\psi$ angles are allowed. When it goes into some specific angle, there are steric clashes.
- using molecular models to test the possible value of dihedral angles.
- only a few dihedral angles for normal natural proteins.
- The result was presented as a plot: Ramachandran Plot.
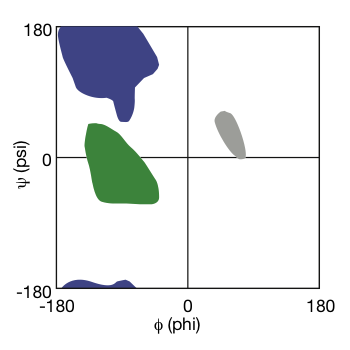 |
|---|
| Ramachandran Plot © HarvardX |
As shown in the Ramachandran plot, the dihedral angle falls into green forms alpha helix, hit in purple forms beta-sheet, or end in the grey area becomes a loop.
Proline break alpha helix
Proline break alpha helix
Alpha helix
In alpha-helix:
- Right-handed helix.
- each turn as 3.6 residues.
- Hydrogen bonds between nitrogen residue and carboxyl residue were formed.
- The side chain is towards us and slightly downwards towards the N-terminals.
Exp: In a partial alpha-helix of ATP synthesis, all hydrophobic residues are in the same face which forms a hydrophobic core against another helix group.
Beta sheet:
string, arrow stands from N terminal to C terminal.
Type: mixed, anti-parallel strands, parallel strands.
When the beta-sheet was formed, the side chains were performed above or below the sheet. Exp: alternating aliphatic and polar residues could form a sheet with one hydrophobic side and one polar side.
loop
Alpha helix and beta-sheets need a series of repetitions to form a structure. If the pattern is not continued, they could be loops. So, all regions of the Ramachandran plot could form loops.
glycine being very flexible, and proline being restricted, but being able to form cis peptide bonds. And so proline and glycine are often found within loop regions of proteins.
Proline is technically an imino acid! This means that the R-group connects to the backbone at nitrogen AND C-alpha. This prevents hydrogen bonding that sustains the secondary structure of an alpha helix.
Prolines are often found at the beginning and the end of alpha-helices. They can also make strong kinks in the helical structure because the N-terminal peptide bond is more constrained.
Tertiary and Quaternary Structure
Both structures are the result of non-covalent bond interactions: Van de Waals, hydrogen bond, and salt bridges.
Tertiary: Arrangement of single peptide
Quaternary: Arrangement of several peptides
Example: alcohols dehydrogenase:
Motif: The smallest secondary structures are assembled in a consistent way. (A commonly repeated arrangement of a few secondary structural elements.) exp: Beta alpha Beta motif:
Domain: One or more motifs can assemble to form a compact globular structure. It is an independent folding unit in a protein. They can fold on their own when you isolate them independently from the rest of the protein. exp: Rossmann fold.
The active site of an enzyme is often located in a loop region which could be shaped both chemically and structurally to provide specifically. And it is often in the crevasses between two domains that are rich in loops.
dehydrogenase 3d model; © Molview.org
In this enzyme, two identify Peptides joined by beta-sheets to form a functional quarternary structure.
Domain-Fold:
Fold: classify protein structure; An arrangement of secondary structural elements of a domain or protein
Fold
Rosman fold
 |
|---|
| © Daniel A. Bochar[1] |
Rossmann fold was a sandwich structure which beta-sheet enveloped by alpha-helix. As you can see in the 3D model above, the Rossmann fold serves as an activity site for alcohol dehydrogenase.
Beta fold: carbonic anhydrase
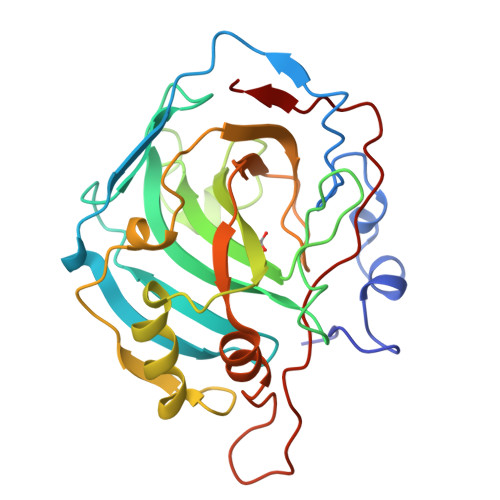 |
|---|
| © PDB:1HCB |
Carbonic anhydrase catalyzes the inter-conversion of carbonic acid, the result of dissolved carbon dioxide and bicarbonate. It is therefore a critical enzyme for maintaining pH balance in the blood.
Alpha helix fold: myoglobin
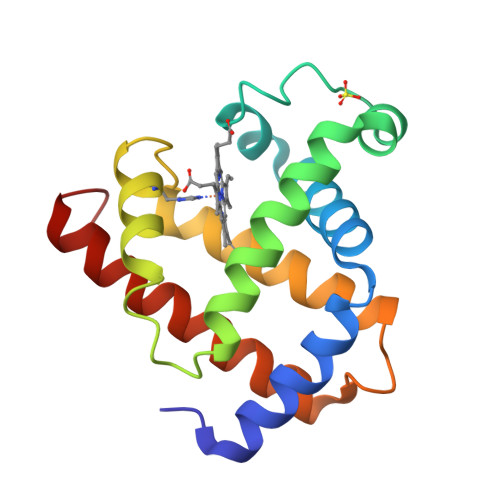 |
|---|
| © PDB:1mdb |
similar sequences have a similar structure,
diversity sequences can still have a similar structure.
All grey background image of molecular formulae comes from PubChem
The side-chain of its pKa is 6.
3D structure model was embedded from molview.org
Daniel A. Bochar, Jona. Freisen, Cynthia V. Stauffacher, Victor W. Rodwell,2.02 - Biosynthesis of Mevalonic Acid from Acetyl-CoA,Editor(s): Sir Derek Barton, Koji Nakanishi, Otto Meth-Cohn,Comprehensive Natural Products Chemistry,Pergamon,1999,Pages 15-44,ISBN 9780080912837,https://doi.org/10.1016/B978-0-08-091283-7.00035-7. ↩︎
Principles of Biochemistry 3 |Amino acid to Protein| Class Notes |HarvardX
https://karobben.github.io/2021/03/24/LearnNotes/edx-biochm-3/








