Vesicular Traffic
- Lodish MCB, 8th ed., pages 631-659.
14.1 Techniques for Studying the Secretory Pathway
14.2 Molecular Mechanisms of Vesicle Budding and
Fusion
14.3 Early Stages of the Secretory Pathway
14.4 Later Stages of the Secretory Pathway
14.1 Studying the Secretory Pathway
- Various labels enable tracking protein movement through the secretory pathway to different destinations.
- Components required for intracellular protein trafficking have been identified by analysis of yeast temperature-sensitive secretory (sec) mutants.
- Cell-free assays for intercompartmental protein transport have defined individual steps of the secretory pathway.
Protein Transport through the Secretory Pathway
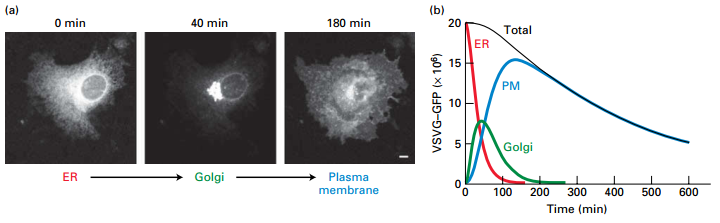
- GFP labeled viral membrane glycoproteins synthesized and transported to host cell membrane by the constitutive secretory pathway.
- Looked at time points to determine cell location.
- At 0 time point, VSV G-GFP retained in ER.
- At 40 min, most concentrated in the Golgi.
- At 180 min, most VSV G-GFP has integrated into and is diffusing within the plasma membrane.
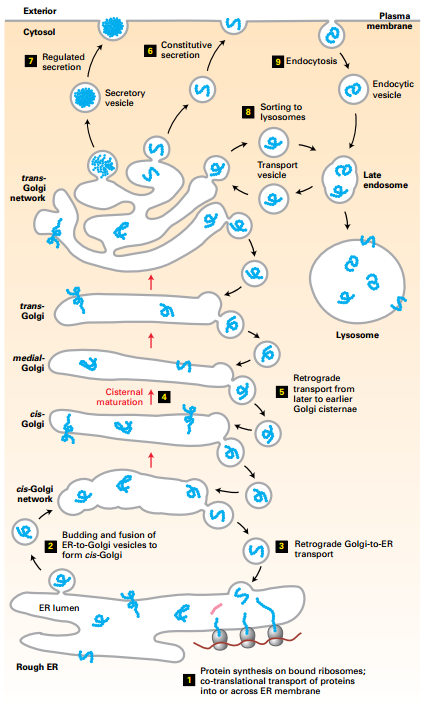
Secretory and Endocytic Pathways
- Transport vesicles transport membrane and soluble proteins from one membrane-bound compartment to another.
- Transport vesicles collect cargo proteins in membrane budding from a donor compartment and deliver cargo by fusing with a target membrane.
- Secretory pathway distributes cargo proteins to final destinations (ER, Golgi, cell surface (including secretion) or lysosomes.
- Cargo buds from the ER, fuse together to form a new cis-Golgi cisternae → move by nonvesicular cisteral maturation, and retrograde vesicle transport.
- Regulated (aggregates) and constitutive secretion.
- Lysosomal proteins bud and fuse with late endosome.
- Endocytic vesicles bud from plasma membrane, delivered to lysosomes via late endosomes.
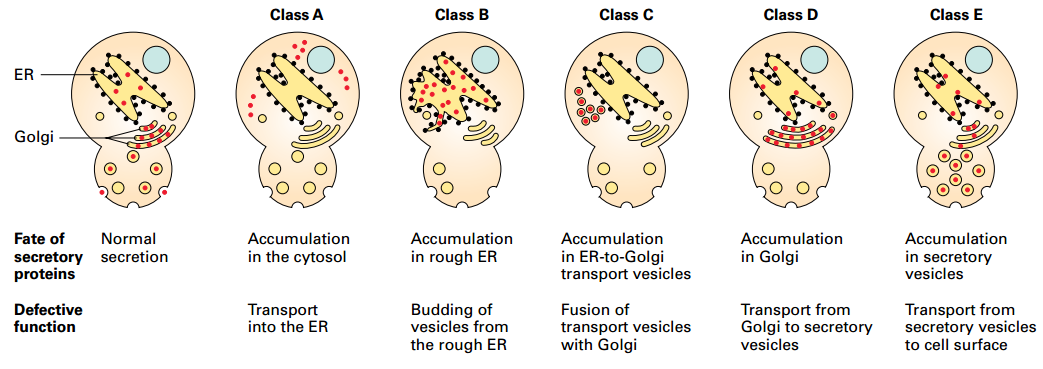
Sec Mutants Identify 5 Stages of Secretory Pathway
- Yeast temperature-sensitive mutants define 5 major stages and individual components involved in targeting and vesicular transport: Cytosol, Rough ER, Vesicles transporting proteins from the ER to the Golgi complex, Golgi cisternae, and constitutive secretory vesicles
- Mutations simultaneously in both class B and class D functions: proteins accumulate in rough ER, not in Golgi → defines order of steps
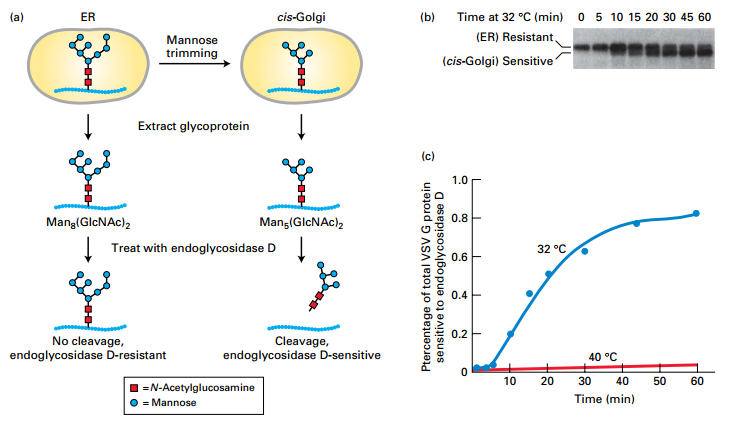
ER to Golgi Transport and Processing
- Yeast temperature-sensitive mutant: At 40°C is retained in ER, not processed in Golgi. At 32°C, transported to Golgi and processed.
- Shows that processing of glycosylation occurs in Golgi.
EXPERIMENTAL FIGURE 14-3 Transport of a membrane glycoprotein from the ER to the Golgi can be assayed based on sensitivity to cleavage by endoglycosidase D.
- Detecting secretory protein carbohydrate modifications by compartment-specific enzymes elucidates trafficking through different compartments.
Experiment:
- Temperature-sensitive VSV G protein – labeled with a pulse of radioactive amino acid during synthesis in cells
- Split cells into two groups –
- Group 1 – Control cells maintained at 40°C – misfolded protein retained in ER
- Group 2 – Experimental cells shifted to 32°C – protein moves through secretory pathway
- Time points – VSV G protein extracted and treated with endoglycosidase D
- (a) Protein movement to cis-Golgi and modification:
- Protein retained in the ER – not modified
- Protein moved from the ER to the cis-Golgi – core oligosaccharide Man8(GlcNAc)2 is trimmed to Man5(GlcNAc)2 by cis-Golgi resident enzymes
- Endoglycosidase D – cleaves the oligosaccharide chains from proteins processed in the cis-Golgi, but not from proteins retained in the ER
- (b) SDS-polyacrylamide gel electrophoresis of the digestion mixtures (Autoradiography only shows labeled VSV G protein in gel.):
- (ER) resistant – uncleaved (larger-slower-migrating)
- (cis-Golgi) sensitive – cleaved (smaller-faster-migrating)
- 5 min – most protein resistant to EndoD cleavage – not yet moved from ER to cis-Golgi ER
- 60 min – most protein cleaved by EndoD cleavage – moved to and modified in the cis-Golgi
- © Percentage of EndoD-sensitive VSV G protein over time:
- Derived from electrophoretic data
- 40°C – little increase n sensitive protein – misfolded proteins retained in ER
- 32°C – most protein moved from ER to cis-Golgi and modified by 30–40 min
Protein Transport from One Golgi Cisternae to Another
- Mutant in cis-Golgi. Cannot process from High mannose glycosylation.
- Wild type processes glycosylation in each of the Golgi cisternae.
- Mixing of Golgi shows cis-Golgi enzyme (N-acetylglucosamine transferase) moved from wild type medial Golgi to mutant cis-Golgi by retrograde vesicle transport during cisteral maturation process.
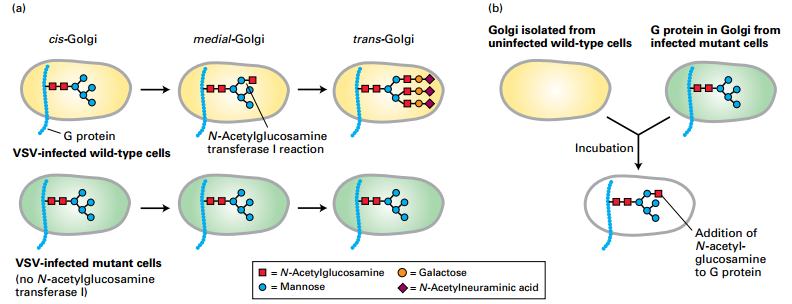
EXPERIMENTAL FIGURE 14-5 A cell-free assay demonstrates protein transport from one Golgi cisterna to another.
- Cell-free transport assays – biochemical dissection of secretory pathway individual steps
Experiment:
- VSV virus infection of cultured mutant cells lacking one of the Golgi enzymes that modify N-linked oligosaccharide chains –fate of the VSV G protein.
- (a) VSV G protein modification in Golgi of intact wild-type and mutant fibroblast cells
- Wild-type cells:
- medial-Golgi – N-acetylglucosamine transferase I adds one N-acetylglucosamine to N-linked oligosaccharides
- trans-Golgi – N-linked oligosaccharides further modified to more complex carbohydrate
- Mutant cells – lack medial-Golgi N-acetylglucosamine transferase I:
- medial-Golgi – no addition of N-acetylglucosamine
- trans-Golgi – no further modification to complex carbohydrate
- G protein reaches the cell surface with a simpler high-mannose oligosaccharide containing only two N-acetylglucosamine and five mannose residues.
- (b) Golgi isolated from VSV-infected mutant cells and noninfected wild-type cells:
- Mutant cell Golgi – contains VSV G protein
- Wild-type cell Golgi – no VSV G protein
- Golgi mixed together
- Result: Some VSV G protein modified by wild-type medial-Golgi N-acetylglucosamine transferase I
- Conclusion: N-acetylglucosamine transferase I – moved by transport vesicles from the wild-type medial-Golgi cisternae to the mutant cis-Golgi during cisternal maturation process.
14.2 Molecular Mechanisms of Vesicle Budding and Fusion
- Three types of coated vesicles mediate protein transport through different pathways.
- Small GTPase proteins direct coat protein polymerization on donor membranes to pinch off vesicles carrying different cargoes.
- Coat shedding exposes Rab and SNARE proteins that target vesicles for fusion with specific target membranes.
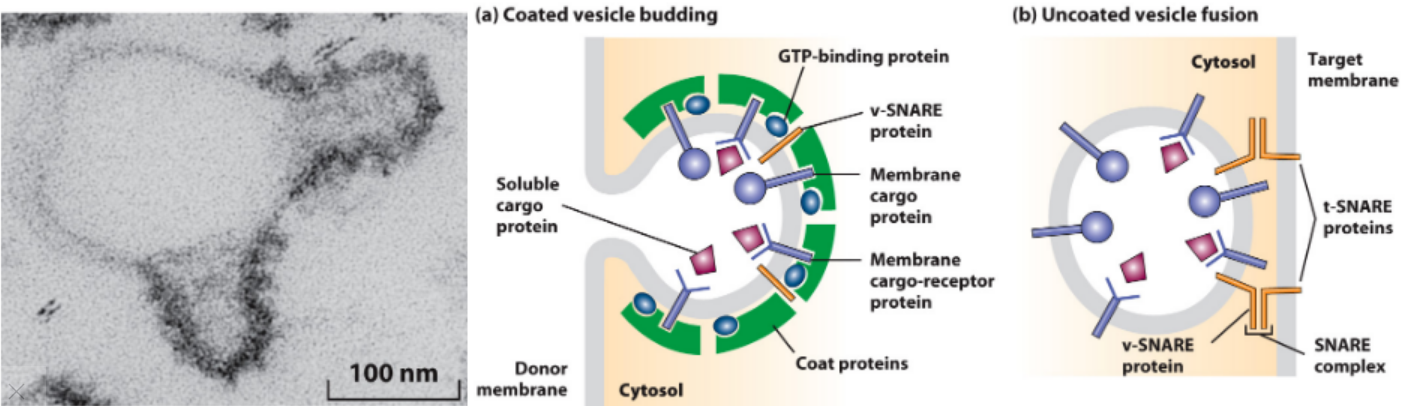
Vesicle Budding and Fusion with a Target Membrane
- Coat proteins bind membrane cargo cytosolic domain.
- Soluble cargo bind receptors, receptor also binds coat protein.
- Vesicle SNARES (v-SNARES) captured in budding vesicle.
- Coat evaginates donor membrane, pinches off coated vesicle.
- Monomeric GTP-binding protein also binds coat protein in GTPbound form. GTP hydrolysis → decoating. G protein also leaves.
- Decoating uncovers v-SNARES, interaction with target membrane t-SNARES for membrane fusion.
Coated Vesicles Involved in Protein Trafficking
- Three major types of transport vesicles: each with a different type of protein coat and monomeric GTPase protein.
| Vesicle Type |
Transport Step Mediated |
Coat Proteins |
Associated GTPase |
| COPII |
ER to cis-Golgi |
Sec23/Sec24 and Sec13/Sec31, Sec16 |
Sar1 |
| COPI |
cis-Golgi to ER Later to earlier Golgi cisternae |
Coatomers containing seven different COP subunits |
ARF |
| Clathrin and adapter proteins* |
trans-Golgi to endosome |
Clathrin + AP1 complexes |
ARF |
|
trans-Golgi to endosome |
Clathrin + GGA |
ARF |
|
Plasma membrane to endosome |
Clathrin + AP2 complexes |
ARF |
|
Golgi to lysosome, melanosome, or platelet vesicles |
AP3 complexes |
ARF |
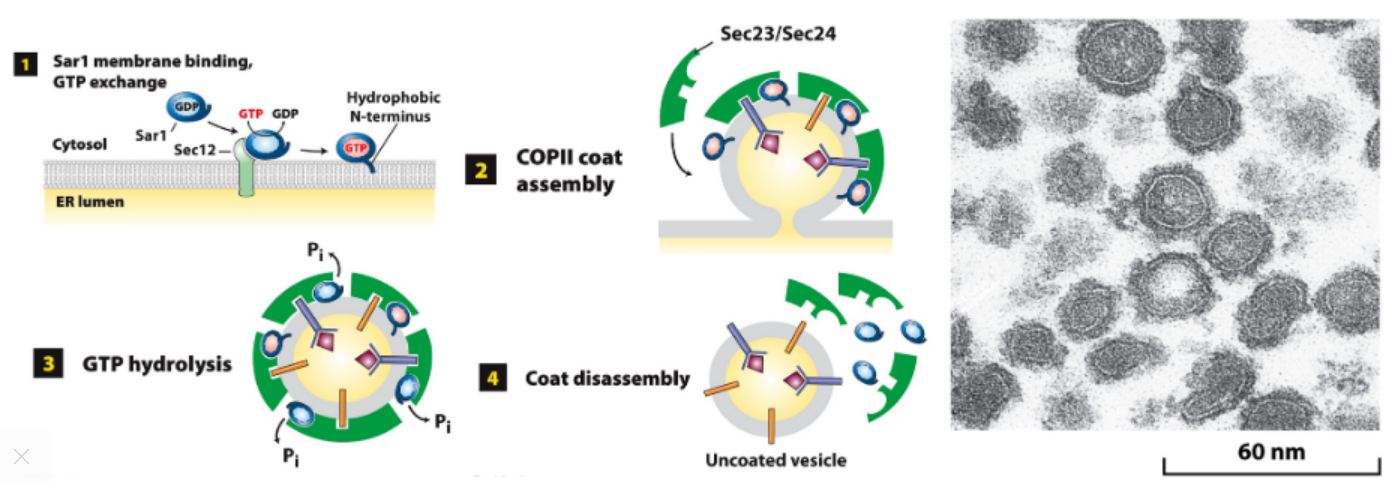
Sar1 Role Assembly/Disassembly of COPII Coat
-
- GEF function: Sar1-GDP → Sar1-GTP, membrane bound.
-
- Sar1-GTP recruits coat proteins, curvature forms bud w/ cargo.
-
- GTP hydrolysis, Sar1-GDP loses affinity for vesicle membrane.
-
- Sar1-GDP not membrane bound, promotes coat disassembly.
- Image: Coated vesicles accumulate during in vitro budding in presence of nonhydrolyzable GTP analog.
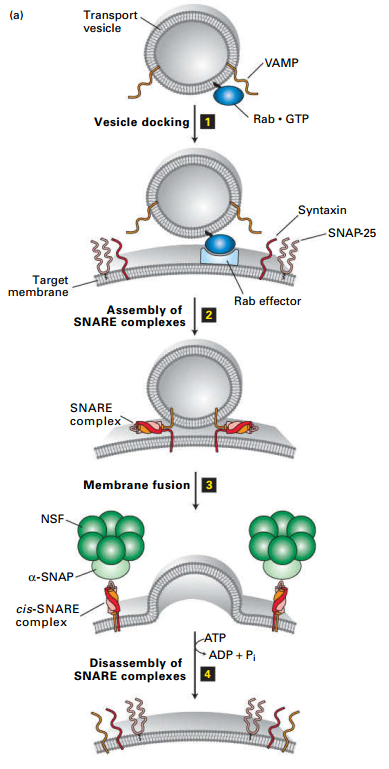
Docking/Fusion of Vesicle w/Target Membrane
- Vesicle Rab binds Rab effector on target membrane.
- v-SNARE forms stable coiled-coil interaction with t-SNAREs (syntaxin and SNAP-25); hold vesicle close to target membrane.
- Fusion of two membrane → drives dissociation of the SNARE complexes (4), freeing SNARE proteins (recycled by NSF – an AAA-family member).
- Not shown: Rab-GTP hydrolyzed to RabGDP – dissociates Rab from the Rab effector.
Targeting Sequences on Cytosolic Regions of Cargo Proteins Bind to Specific Coat Proteins
| Signal Sequence* |
Signal-Bearing Protein |
Proteins with Signal |
Vesicles That Incorporate Signal Receptor |
| LUMINAL SORTING SIGNALS |
|
|
|
| Lys-Asp-Glu-Leu (KDEL) |
ER-resident soluble proteins |
KDEL receptor in cis-Golgi membrane |
COPI |
| Mannose 6-phosphate (M6P) |
Soluble lysosomal enzymes after processing in cis-Golgi |
M6P receptor in trans-Golgi membrane |
Clathrin/AP1 |
| CYTOPLASMIC SORTING SIGNALS |
|
|
|
| Lys-Lys-X-X (KKXX) |
ER-resident membrane proteins |
COPI α and β subunits |
COPI |
| Asn-Pro-X-Tyr (NPXY) |
LDL receptor in plasma membrane |
AP2 complex |
Clathrin/AP2 |
14.3 Early Stages of the Secretory Pathway
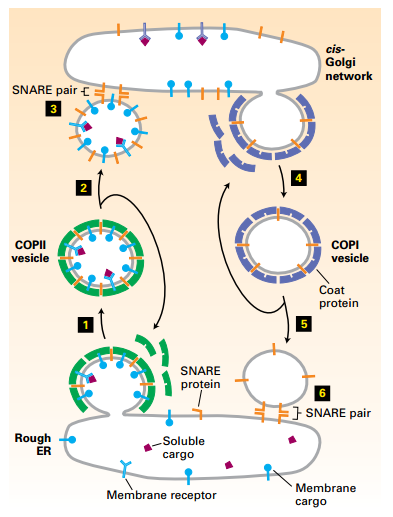
- COPII-coated vesicles transport newly synthesized proteins containing Golgi-targeting sequences in their cytosolic domain or bound to such proteins from the rough ER to the cis-Golgi (anterograde direction).
- COPI-coated vesicles transport vesicles carrying ER/Golgi-resident proteins in the retrograde direction, which supports Golgi cisternal maturation.
Forward (anterograde) transport: COPII vesicles ER → cis-Golgi transport.
- Step 1: COPII coat assembles.
- Step 2: COPII coat disassembly.
- Step 3: Membrane fusion
Reverse (retrograde) transport: COPI
vesicle cis-Golgi to ER transport.
- Cargo: recycles membrane bilayer, v-SNAREs, and missorted ERresident proteins.
- Step 4: COPI coat assembles.
- Step 5: COPI coat disassembly.
- Step 6: Membrane fusion.
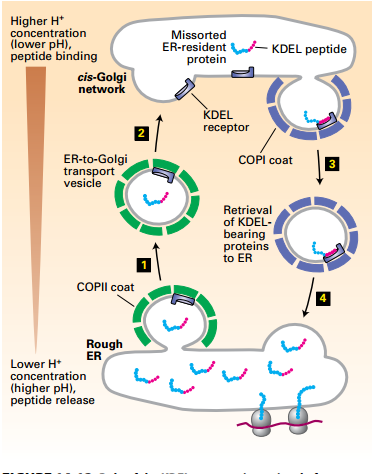
KDEL Receptor Retrieval of ER-Resident Luminal Proteins from the Golgi
- Soluble ER proteins contain KDEL (LysAsp-Glu-Leu) ER-targeting sequence. May become missorted to cis-Golgi: Nonspecifically incorporated into COPII vesicle fluid phase.
- cis-Golgi to ER retrieval mechanism: cis-Golgi acidic pH promotes receptorKDEL interactions.
- Higher pH of ER → dissociates ER resident KDEL protein from the KDEL receptor. Retrieval system prevents depletion of ER luminal proteins.
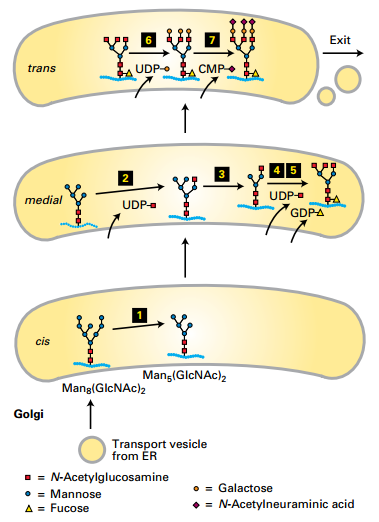
trans-Golgi:
- N-acetylneuraminic acids added (7).
- Galactoses added (6).
medial-Golgi:
- Fucose added (5).
- Mannoses removed (3).
- N-acetylglucosamines (GlcNAc) added (2 and 4).
cis-Golgi:
- Mannoses removed (1).
Anterograde transport occurs by cisternal maturation.
- Golgi cisternae have different enzymes.
14.4 Later Stages of the Secretory Pathway
- The trans-Golgi network sorts proteins into vesicles targeted for different destinations.
- Lysosomal enzymes bear M6P residues that are recognized by M6P-receptors and delivered by a clathrin-coated vesicle pathway to lysosomes.
- Regulated secretory proteins are concentrated and stored until secretion is signaled; constitutively secreted proteins are continuously delivered to the plasma membrane.
- Some proteins are processed into mature form after leaving the trans-Golgi network.
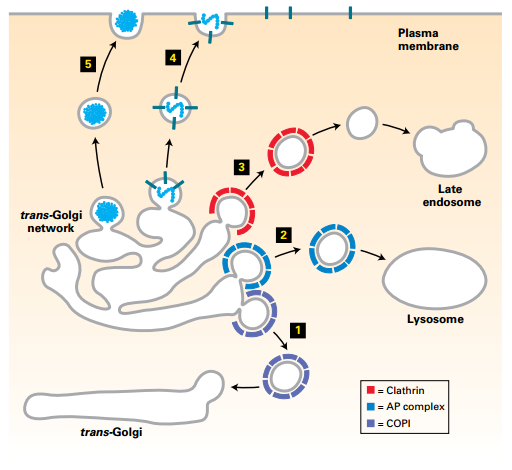
Vesicle Trafficking from trans-Golgi Network
- (5) Regulated secretory vesicles (unknown coat).
- (4) Constitutive secretory vesicles (unknown coat).
- (3) Clathrin-coated (+AP2) vesicles transport to late endosomes.
- (2) AP complex vesicles transport directly to lysosomes.
- (1) COPI vesicles: retrograde to trans-Golgi (cisternal progression).
- Distal sorting trans-Golgi network: transport vesicles to plasma membrane, endosomes, and lysosomes.
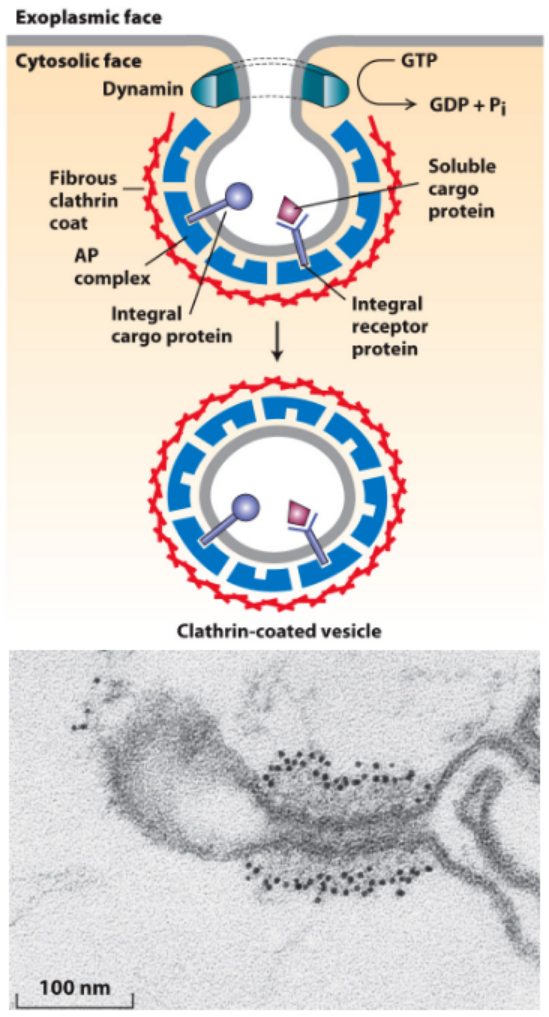
Dynamin Pinching Off of Clathrin-Coated Vesicles
- Dynamin:
- Required for pinching off of clathrin-coated vesicles
- Polymerizes around vesicle neck
- Hydrolyzes GTP – conformational change coupled to membrane fusion and vesicle release from the donor membrane.
- GTP hydrolysis by dynamin is required for the pinching off of clathrin-coated vesicles (cells incubated with GTP-γ-S nonhydrolyzable analogue of GTP).
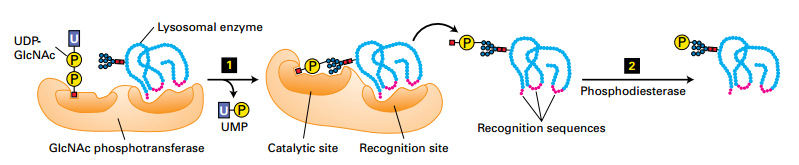
M6P signal directs newly synthesized enzymes to lysosomes.
- Step 1: cis-Golgi-localized N-acetylglucosamine (GlcNAc) phosphotransferase transfers a phosphorylated GlcNAc group to carbon 6 of one or more mannose residues.
- Step 2: Phosphodiesterase removes the GlcNAc group, leaving a 6-phosphorylated mannose residue on the lysosomal enzyme.
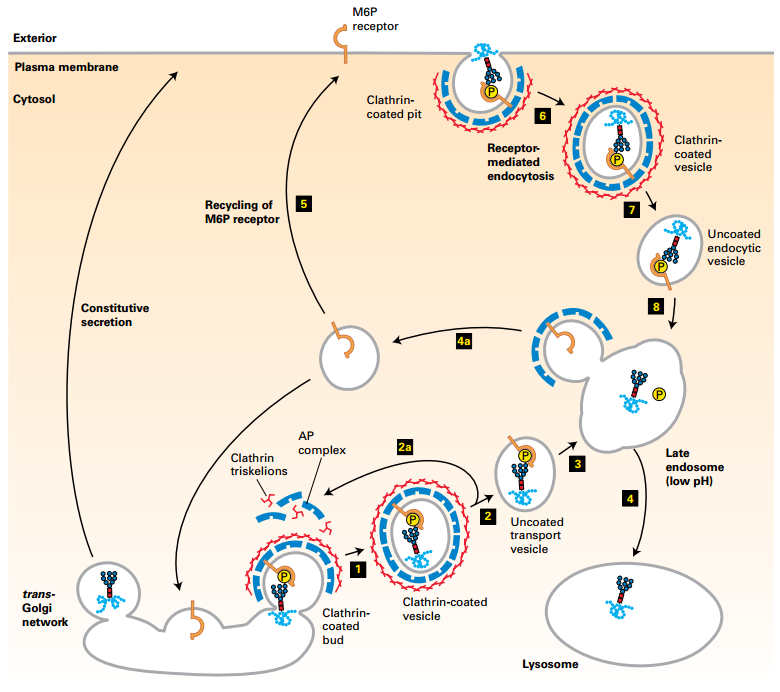
Trafficking of Lysosomal Enzymes from trans-Golgi Network and Cell Surface to Lysosomes
- M6P receptors in trans-Golgi membrane recruit M6P-lysosomal enzymes into clathrin/AP1-coated vesicles (1).
- Clathrin coat disassembles (2). Coat proteins recycled (2a).
- Vesicle fuses with late endosome compartment (3). Acidic pH dissociates M6P-enzyme from receptor.
- Late endosome fuses with a lysosome (4). M6P receptors recycled (4a).
- Some receptors are delivered to the cell surface (5a). Plasma membrane M6P receptors (5) capture missorted M6P-enzymes into clathrin-coated vesicles in the receptor-mediate endocytosis pathway (6). Deliver enzyme to endosome/lysosome (7-8)
- Lack of N-acetylglucosamine phosphotransferase activity.
- Lysosomal enzymes lack M6P targeting signal → constitutively secreted (5a).
- Continued accumulation of undigested lysosomal contents → forms “inclusions bodies”, may eventually kill cells.
- Causes developmental, physiological, and neurological abnormalities.
- Referred to as Mucolipidosis.
- In vitro “cure” – M6P-enzymes added to I-cell fibroblasts – internalized by endocytosis pathway and function on arrival in lysosomes. VERY EXPENSIVE.
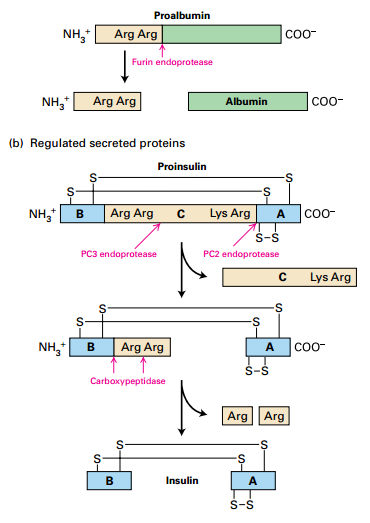
Proteolytic Processing of Proproteins in Constitutive / Regulated Secretory Pathways
- Proproteins matured after leaving trans-Golgi.
- (a) Proalbumin processing – typical of proteins in constitutive secretory pathway: Furin endoprotease cleaves peptide.
- (b) Insulin processing – typical of proteins in regulated secretory pathway:
- PC2 and PC3 endoproteases – cleave central region of insulin.
- C peptide secreted with insulin. High C peptide levels → kidney problems or insulinoma. Low C peptide → low insulin (T1 diabetes).
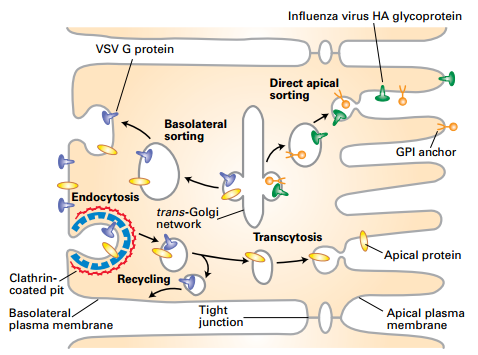
Sorting Proteins Destined for Apical and Basolateral Membranes of Polarized Cells
- VSV G protein only on the basolateral membrane (VSV viruses bud only from the basolateral membrane.) Serosal Mucosal
- Influenza HA glycoprotein only on the apical membrane (Influenza viruses bud from only from the apical membrane). Also GPI proteins in some cell types
- IgA internalized at basolateral membrane and sorted to apical membrane by transcytosis
- Some hepatocyte apical membrane proteins sorted initially to basolateral membrane, endocytosed and transcytosed through endosomes to the apical membrane.