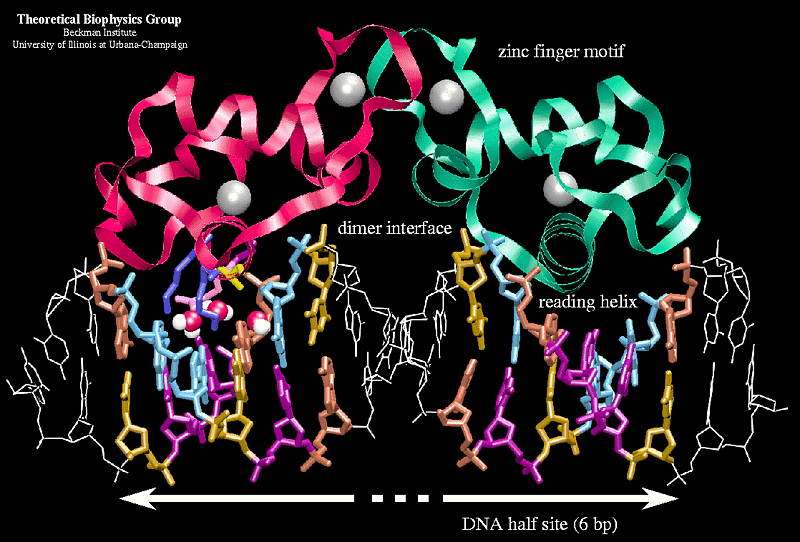14 Nuclear Receptors|Tulane
Nuclear Receptors
I. History of Nuclear Receptor Research
II. The Nuclear Receptor Superfamily and Classification
III. Structure of Nuclear Receptors
IV. Mechanisms of Nuclear Receptor Signaling
V. Nuclear Receptors in Health and DiseasesFather of
- 1966 Nobel Laureate30+ years of nuclear receptor research
Evans RM and Mangelsdorf DJ. Cell 2014 157, 255-266Discovery of RXR: the Big Bang in NR
research
Evans RM and Mangelsdorf DJ. Cell 2014 157, 255-266
Nuclear receptor research: recent topics
• Transcriptome and proteome analysis
• Crosstalk of nuclear receptor with other signaling pathways: PI3k/AKT, STATs, mTOR, PKA, PKC
• Epigenetic regulation of NRs: non-coding RNAs, histone modificication
• Non-canonical NRs: mutants, splice variants
• Non-genomics actions of nuclear receptors
• Therapeutic targeting: SERMs, SARMs, etc
Terminology
• Ligand: the signaling molecule. It may be a hormone, a growth factor/cytokine, a steroid, a polypeptide, or other type of molecules. The ligand has no activity of its own, it must bind to a macromolecule which is known as a receptor.
• Receptor: protein molecules that bind to ligand with high specificity. When activated by the ligand, the receptor induces changes in the target cells/tissues in which it is expressed.
• Hormone response element (HRE): the specific motif on the DNA to which the receptor binds.
• Dimerization: one protein molecule forms a complex with another, usually via non covalent bonds. The protein molecules can be the same (homodimer) or different (heterodimer).
The mode of signaling is determined by the chemical nature of the ligand
• Lipophilic or hydrophobic ligands such as steroids can pass through the cell membrane and thus use intracellular receptors.
• Hydrophilic ligands such as peptides and growth factors cannot pass through the cell membrane and thus use cell surface receptors.
Hormone signaling
Nuclear Receptor: Classical Definition
Nuclear receptors (NRs) are a family of structurally similar, ligand-activated transcription factors that reside within the cells. Upon binding to ligands, NRs exert their functions in the nucleus and regulate the expression of genes with a wide range of physiological functions, such as embryonic development, cell proliferation, differentiation, metabolism, cell death, etc.
Type I Nuclear Receptor (Steroid Hormone Receptors)
- Including ER, PR, AR, GR, and MR
- Ligand-dependent transcription factors
- Unliganded receptors reside in the cytoplasm, form a complex with chaperones (heat shock proteins and immunophilins)
- After binding to ligands, translocate to the nucleus and bind to DNA as homodimers.
- Hormone response elements (HREs) are inverted repeats.
Steroidogenesis Pathway
Adrnal Cortex
- Aldosterone
- Stimulates renal reabsorption of Na⁺ and excretion of K⁺.
- Cortisol
- Increased Glucongenesis
- Anti-inflammatory
- Protein breakdown in muscle
Ovary
- Estrogens
- Control menstrual cycle
- Promote development of female secondary sex characteristics.
- Progesterone
- Secretory phase of uterus and mammary glands.
- Implantation of maturation of fertilized ovum.
Testes
- Testosterone
- Stimulate spermatogenesis
- Promotes development of male secondary sex characteristics
- Promotes anabolism
- Masculinization of the fetus
Type II Nuclear Receptor (RXR heterodimers)
- including receptors for retinoid acid (RAR), vitamin D (VDR), thyroid hormone receptor (TR), and peroxisome proliferator-activated receptor (PPAR)
- Ligand-dependent transcription factors
- Unliganded receptors are in the nucleus, bound to DNA in the promoter of target genes, interact with co-repressors, and repress basal transcription
- bind to DNA as heterodimers with RXR
- in the presence of ligands, undergo conformational change, dissociate from co-repressors and recruit co-activators
- HREs are direct repeatsLigands for Type II Nuclear Receptors
Ligands for Type II Nuclear Receptors
Type I: ERl; AR; GR
Type II: RAR; TR; VD
- thyroid hormones
- retinoids
- vitamin D
Direct repeats of Type II HREs
Same repeat patterns but different length in the insertion:
VD3RE:3n
RARE:5n
Type III Nuclear Receptors (Orphan Receptors)
- including Rev-Erb, ROR, ERR, NGFI-B, SF-1
- In general, are located in the nucleus, bound to DNA and repress basal transcription
- Activation through signaling pathways or unknown ligands
- Bind to DNA either as monomers, or homodimers, or heterodimers with RXR
Dimeric orphan recptors and monomer orphan recptors
Nuclear receptor classification summary
| Type I | Type II | Type II | |
|---|---|---|---|
| Ligands | Known | Kown | Unknown or No |
| Location w/o ligands | Cytoplasm | Nucleus | Nucleus |
| Nuclear translocation | Y | N | N |
| Dimerization | Homodimer | Heterodimer with RXR | Homodimer, Monomer, or heterodimer with RXR |
| HRE | Inverted repeats | Direct Repreats | Direct repeat or half site |
Structure of Nuclear Receptors
translocation Yes No No
Dimerization Homodimer Heterodimer
with RXR Homodimer,
monomer, or
heterodimer
with RXR
HRE Inverted
repeats Direct repeats Direct repeat or
half siteLecture Outline
I. History of Nuclear Receptor Research
II. The Nuclear Receptor Superfamily and
Classification
III. Structure of Nuclear Receptors
IV. Mechanisms of Nuclear Receptor Signaling
V. Nuclear Receptors in Health and Diseases
Domain Organization of Nuclear Receptors
A/B → C → D → E → F
A/B: N-terinal domain
C: DNA binding Domain (DBD)
D: Hinge domain
E: ligands bindg domain
F: C-terminal
Sequence homology of Nuclear Receptors
DNA binding domain: most conserved one
Degree of similarity: DBD > LBD > NTD
N Terminal Domain (NTD)
- Highly variable among NRs. Unstructured or structurally diverse.
- Type I NRs have large NTDs (400-600 a.a.); Nonsteroid receptors have much shorter NTDs (VDR 24 a.a.)
- Contains Activation Function -1 (AF-1) domain, which is responsible for ligand-independent activation.
- AF-1 synergizes with AF-2 in the LBD.
- NTD is involved in co-regulator recruitment. AR has ~150 co-regulators interacting with AF-1.
DNA Binding Domain (DBD)
The most conserved domain among NRs. Contains two zinc- finger motifs. Responsible for binding to hormone response elements (HREs).
Zinc Finger Motifs in DBD
- Evidence for role of zinc fingers in receptor function:
- Site-directed mutagenesis destroys DNA-binding
- Chelation of zinc in vitro destroys DNA-binding
- 1 st zinc finger has P-Box (Proximal Box), critical for sequence recognition and contacting the major groove of DNA.
- 2 nd zinc finger has D-Box (Distal Box), which is involved in dimerization
Evans R M Molecular Endocrinology
Where are the P-boxes and D-boxes?
Hinge domain
–K--------RK----RK–
Between DBD and LBD, confers flexibility to NRs. Also contains Nuclear Localization Signal (NLS), which is critical for nuclear translocation of Type I NRs. NLS motifs are highly conserved in type I receptors.
Ligand Binding Domain (LBD)
Moderately conserved among in sequence, but highly conserved in secondary structure. There are 12 α-helices forming the ligand-binding pocket.
H12
H3
ligand binding is not static, is dynamic.
LBD contains the ligand-dependent activating function-2 (AF-2) domain, which is involved in recruiting coactivators.
Positioning of H12 determines the activity of NR
NCoA: nuclear receptor coactivator (LxxLL)
NCoR: nuclear receptor corepressor (LxxIIxxxL)
Ligand-induced conformation change in the LBD
Ligand binding leads H12 movement
H12 conformation change is used in drug design
DES: diethylstilbestrol
Fraydoon Rastinejad et al. J Mol
Block the movement of the H12, hinder the contact between H12 and ligands.
Drug design based on H12 conformation change
Mechanisms of Nuclear Receptor Signaling
SKey Steps in Type I NRs Signaling
• Ligand binding
• Receptor dimerization
• nuclear translocation
• DNA binding
• Recruitment of co- regulators (coactivators and corepressors)
Chaperones and Co-chaperones for Type I Receptors
- Stabilize the receptors, preventing degradation
- Maintain the proper conformation of the LBD to facilitate ligand binding.
- Play an important role in nuclear translocation of SRs
Chaperones maintain the conformation of NRs for ligand-binding
Transport of proteins across the nuclear membrane
NLS: nuclear localization signal; NES: nuclear export signal
LMB: leptomycin B, inhibitor of nuclear export
Nucleocytoplasmic Shuttling of Type I Nuclear Receptors
- Type I NRs cannot diffuse through the nuclear pore complex due to their sizes (> 40 KDa)
- Nuclear translocation is a tightly regulated process.
- Depending on the receptor and physiological settings, the nuclear translocation of Type I NRs can be ligand-dependent or –independent.
- The active transport of NRs always dependents on the presence of nuclear localization signals (NLSs).
NLSs of Type I Receptors NTD
• Bipartite structure: two clusters of basic amino acids separated by a spacer
• The first cluster is in the DBD, the second in the hinge domain
• NLS activity is also found in the LBD and NTD
Two-step Model for Type II Receptor Activation
Nuclear Receptor Co-regulators
- Chaperones and co-chaperones: Hsp90, Hsp70
- Coactivators and corepressors
- Histone modifying enzymes: HATs, HDACs
- Recruiters of basal transcription machinery
Histone Acetylation/deacetylation by Coregulators
Nuclear Receptor Coactivators and Corepressors
Steroid Receptor Coactivator Family (SRC-1, 2, 3)
Not to be confused with Src kinase!
Corepressors:
Nuclear receptor CoRepressor (NCoR)
Silencing Mediator for Retinoid or Thyroid-hormone
receptors (SMRT)
Complexity of Nuclear Receptors Signaling
- Genomic actions
- Ligand-dependent, HRE-dependent
“classical mechanism” - Ligand-dependent, HRE-independent
“transcriptional crosstalk” - Ligand-independent
- Ligand-dependent, HRE-dependent
- Non-genomic actions
- Non-genomic to genomic signaling
Nuclear Receptors Signaling: multiple modes
NUCLEUSRapid effects of hormones
- Ca 2+ influx and PKC activation: progesterone, aldosterone, VitD3
- Vasodilation: estrogen
Evidence for Non-genomic Actions of Nuclear Receptors
- These effects are too rapid (seconds to minutes) to be explained by transcription and protein synthesis
- These effects cannot be blocked by transcriptional inhibitors (actinomycin D) or protein synthesis inhibitors (cycloheximide)
- These effects can be elicited by nuclear receptors that lack or have inactive transcriptional activation domains
- Some effects can be reproduced by using steroid hormones coupled to membrane- impermeable molecules.
Membrane associated Nuclear Receptors
- classical NRs are associated with the membrane in the following manners:
- tethered to the membrane by palmitoylation
(ER, AR, PR) - docked in membrane microdomains (caveolae or lipid rafts)
- in close association with specific G-protein
couple receptors (GPCRs)
- tethered to the membrane by palmitoylation
Non-genomic Actions of Nuclear Receptors
- ERs have been found in caveolae and activate endothelial nitric oxide synthase (eNOS) by phosphorylation
Non-genomic actions of ARConvergence of Genomic and Nongenomic actions
Convergence of Genomic and Nongenomic actions
Nuclear Receptors in Health and Diseases
- Physiology Roles of Nuclear Receptors
- Discovery of RXR: the Big Bang in NR research
- Sites of Androgen Receptor Action
Androgen Receptor in Health and Disease
- Male Sex Development and Reproduction.
- Androgen Insensitivity Syndrome (AIS). Caused by mutations in AR, leading to truncated receptor or altered ligand affinity. Symptoms are male sexual development is deterred or absent.
- Kennedy’s Disease. a.k.a. spinal and bulbar muscular atrophy (SBMA), is caused by increased number of CAG repeats in exon 1 of AR. Affected individuals have progressive muscular weakness, cramps and witching in the limbs. Males have testicular atrophy, reduced fertility, and excessive development of the mammary glands. Females are usually carriers.
Androgen Receptor in Health and Disease
- Cancer. Signaling through AR are critical for all stages of prostate cancer, including castration-resistant prostate cancer (CRPC). Mechanisms for AR activation in CRPC have been hot topics of investigation. Also play a role in cancers of the breast, liver, kidney, bladder
- Brain Development and Behavior. Widespread expression of AR is found in the mammalian brain, playing a role in brain development, e.g. white matter growth during adolescence, brain masculinization.
- Muscle Growth.
- Bone homeostasis and Osteoporosis.
- Immune Function. Maturation of B- and T- lymphocytes.
- Regulation of glucose metabolism and diabetes. Controls insulin secretion and sensitivity.
Sites of Estrogen Receptor Action
Estrogen Receptor in Health and Disease
- Female Development and Reproduction.
- Skeletal Homeostasis and Osteoporosis. ER regulates bone homeostasis in both male and female.
- Breast Cancer. Estrogen promotes the proliferation of mammary epithelial cells. Selective Estrogen Receptor Modulator (SERM) and aromatase inhibitors are used in the treatment of breast cancer.
- Ovarian and Endometrial Cancer.
- Neuroprotective Effects. Protection against neuro- degenerative diseases, such as stroke, Alzheimer’s Disease, Parkinson’s Disease.
Expectations
- Domain structure of NR and function of each domain
- Types of NRs
- Diversity of NR signaling mechanisms
- Coregulators
What type of nuclear receptor?
Additional Readings
Bain D. et al. Nuclear Receptor Structure: Implications for
Function. Annu. Rev. Physiol. 2007, 69:201–20
Bjornstrom L. and Sjoberg M. Mechanisms of Estrogen
Receptor Signaling: Convergence of Genomic and
Nongenomic Actions on Target Cells. Molecular
Endocrinology 2005, 19:833-842.
Ronald M. Evans and David J. Mangelsdorf. Nuclear
Receptors, RXR, and the Big Bang. Cell 2014, 157:255-266
14 Nuclear Receptors|Tulane
https://karobben.github.io/2021/11/03/LearnNotes/tulane-cellbio-14/