Liver and Steroid Hormones—Can a Touch of p53 Make a Difference?
Liver and Steroid Hormones—Can a Touch of p53 Make a Difference?
Cite: Charni-Natan M, Aloni-Grinstein R, Osher E and Rotter V (2019) Liver and Steroid Hormones—Can a Touch of p53 Make a Difference? Front. Endocrinol. 10:374. doi: 10.3389/fendo.2019.00374
General Information
Background
Liver:
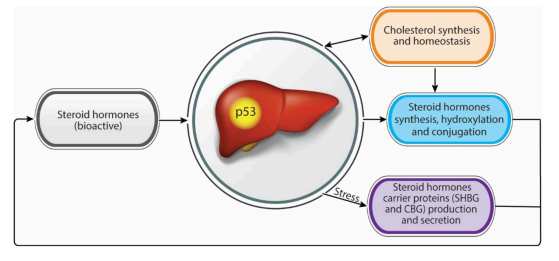
© Charni 2019
Abstract
- Liver serving as a hormonal secretory gland.
- Steroid hormones is important
- Liver is important to Steroid hormones homeostasis
- P53 has impact on Liver
- Hypothesis: P53-> Liver -> Steroid hormones -> Diseases
- New way to diseases treatment (Maybe)
Introduction
P1: BG
Steroid hormones:
- reproductive
- metabolic homeostasis
Steroid hormones synthesis
- derived from cholesterol
- synthesized by adrenal cortex, gonads, and placenta.
Five groups: (based on nuclear receptor)(1–5)
- mineralocoriticoids
- glucocorticoids
- androgens
- estrogens
- progestogens
P53
- Responding for stress
- Regulating hormones expression
This discuss is focus on bidirectional relationship between different steroid hormones and liver and dependently on p53.
Steroid Hormones Homeostasis Regulation by the Liver
Cholesterol
Cholesterol Genesis(15)
- de-novo production
- hydrolysis of stored cholesterol
- interiorization of plasma membrane cholesterol
- from LDL and HDL
Metabolism
Metabolism(16, 17)
- 5αR1 (liver enzyme 5α-reductase 1)
- 5αR1-KO mice exhibited augmented mRNA levels of various hepatic metabolic regulators genes (e.g., Acc1, Agpat2, Cpt2, and Dgat2) in comparison to WT mice(18).
- CYP (cytochromes P450 enzymes)
- metabolism of many drugs and lipophilic compounds(19)
- CYP3A4, CYP19, CYP2C2B1, and CYP2C11 are the liver CYPs and take part in steroid hormones hydroxylation and processing
- CYP3A4(20,21)
- hydroxylases several steroids such as cortisol, androstenedione, testosterone, and progesterone
- CYP19 (Aromatase)(10)
- transforms androgens to estrogens by the removal of C19 carbon and the aromatization of the steroid A ring.
- CYP2C11 and CYP2B1(10)
- regulate hydroxylation of testosterone
- Nuclear receptors complexes
- NRC such as PXR, VDR, RXR were also found to bind CYP3A4 chromatin and affect its expression (23, 24)
Steroids Conjunction
- exerted by such as sulfotransferare and uridine diphosphate-glucuronosyltransferases (UGT)
- ransfer the steroid hormones into higher polarity metabolites that are better suited to be excreted from the body(10).
- The sulfotransferases that are expressed by hepatic cells and are related to steroids conjugations are HSST, EST, SULT 2A1, and SULT 1E1 (25).
- be regulated by androgens, GCs, and nuclear receptors such as PXR(27).
- sulfotransferase inhibition
UGTs
- Two subfamilies
- UGT2B
- mainly expressed in the liver and is related the processing of steroid hormones(28).
- UGT2B
- UGT
- it induce glucuronidation of steroids, a process that interrupts steroids activity, and enables their elimination.
- Tegulated by several xenobiotics compounds (e.g., PCN, PB), which were reported to increase their mRNA expression levels in rats’ livers (29).
Transportation
As steroids, these hormones are lipophilic thus, when secreted into the blood stream they need to be bound to carrier proteins
- SHBG (Sex hormone binding globulin)
- CBG (Corticosteroid binding globulin)
- This carriers are glycoproteins which are mainly secreted by liver.(30, 31).
- Carrier proteins bind steroids and turn them to be biologically inactive (32).
- effect different molecular pathways and signaling such as apoptosis (33, 34)
- Regulation:
Glucocorticoids (GC)
Glucocorticoids are vital endocrine regulators of homeostasis and adaptation to environmental variations.
potent anti-inflammatory and immunosuppressive agents(40)
- Cortisol(42)
- a main glucocorticoid secreted by the adrenal cortex
- essential for immune system, vascular tone maintance
- etc
Played a major role during stress and severe illness by increasing cardiac output and vascular tonus and decreasing pro-inflammatory cytokines release(43,44) - under the control of: (42)
- HPA (hypothalamus-pituitary-adrenal )
- ACTH (adrenocorticotropic hormone)
- CRH (corticotropin-releasing hormone)
- low-density lipoproteins (LDL) cholesterol insufficiency impair the production of cortisol (45)
NAFLD (Non-alcoholic fatty liver disease)
Metabolic disorder, characterized by hepatic steatosis, the presence of free fatty acids or triglycerides in the liver(46).
- Mice:
- high fat diets & chronically elevated GC(47)
- Patients:
- elevated GC levels(48)
(Skip)
Mineralocorticoids
synthesized by the adrenal cortex that influence salt and water balance.
(Skip)
Androgens
Androgens are the principal male sex hormones that regulated masculinizing (雄性化作用) effects and male sexual behavior.
- DHT(dehydroepiandrosterone)
- it can bind androgen receptor(AR) as well as testosterone(82)
Estrogens
Estrogens are female principal sex hormone.
- E2 (17β estradiol) is essential for human
…
Citation
-
1. Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones–a focus on rapid, non-genomic effects. Pharmacol Rev. (2000) 52:513–56.
-
2. Becker KL. Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins (2001).
-
3. Holst JP, Soldin OP, Guo T, Soldin SJ. Steroid hormones: relevance and measurement in the clinical laboratory. Clin Lab Med. (2004) 24:105–18. doi: 10.1016/j.cll.2004.01.004
-
4. Frye CA. Steroids, reproductive endocrine function, and affect. A review. Minerva Ginecol. (2009) 61:541–62.
-
5. Birzniece V. Hepatic actions of androgens in the regulation of metabolism. Curr Opin Endocrinol Diabetes Obes.(2018) 25:201–8. doi: 10.1097/MED.0000000000000405
-
15. Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular
processing and utilization for biosynthesis of steroid hormones. Nutr Metab.
(2010) 7:47. doi: 10.1186/1743-7075-7-47 -
16. Thigpen AE, Silver RI, Guileyardo JM, Casey ML, Mcconnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J Clin Invest. (1993) 92:903–10. doi: 10.1172/JCI116665
-
17. El-Awady MK, El-Garf W, El-Houssieny L. Steroid 5α reductase mRNA type 1 is differentially regulated by androgens and glucocorticoids in the rat liver. Endocr J. (2004) 51:37–46. doi: 10.1507/endocrj.51.37
-
18. Livingstone DE, Di Rollo EM, Mak TC, Sooy K, Walker BR, Andrew R. Metabolic dysfunction in female mice with disruption of 5α-reductase 1. J Endocrinol. (2017) 232:29–36. doi: 10.1530/JOE-16-0125
-
19. Zimniak P, Waxman DJ. Liver cytochrome P450 metabolism of endogenous steroid hormones, bile acids, and fatty acids. In: Schenkman JB, Greim H, editors. Cytochrome P450. Berlin, Heidelberg: Springer Berlin Heidelberg (1993) p. 123–44. doi: 10.1007/978-3-642-77763-9_8
-
20. Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 β-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. (1988) 263:424–36. doi: 10.1016/0003-9861(88)90655-8
-
21. Niwa T, Murayama N, Imagawa Y, Yamazaki H. Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev. (2015) 47:89–110. doi: 10.3109/03602532.2015.1011658
-
10. You L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem Biol Interact. (2004) 147:233–46. doi: 10.1016/j.cbi.2004.01.006
-
23. Wang K, Chen S, Xie W, Wan YJ. Retinoids induce cytochrome P450 3A4 through RXR/VDR-mediated pathway. Biochem Pharmacol. (2008) 75:2204–13. doi: 10.1016/j.bcp.2008.02.030
-
24. Istrate MA, Nussler AK, Eichelbaum M, Burk O. Regulation of CYP3A4 by pregnane X receptor: The role of nuclear receptors competing for response element binding. Biochem Biophys Res Commun. (2010) 393:688–93. doi: 10.1016/j.bbrc.2010.02.058
-
25. Strott CA. Sulfonation and molecular action. Endocr Rev. (2002) 23:703–32. doi: 10.1210/er.2001-0040
-
26. Strott CA. Sulfonation and molecular action. Endocr Rev. (2002) 23:703–32. doi: 10.1210/er.2001-0040
-
27. Runge-Morris M, Wu W, Kocarek TA. Regulation of rat hepatic hydroxysteroid sulfotransferase (SULT2-40/41) gene expression by glucocorticoids: evidence for a dual mechanism of transcription
-
28. Girard C, Barbier O, Veilleux G, El-Alfy M, Belanger A. Human uridine diphosphate-glucuronosyltransferase UGT2B7 conjugates mineralocorticoid and glucocorticoid metabolites. Endocrinology. (2003) 144:2659–68. doi: 10.1210/en.2002-0052
-
29. Vansell NR, Klaassen CD. Increase in rat liver UDP glucuronosyltransferase mRNA by microsomal enzyme inducers that enhance thyroid hormone glucuronidation. Drug Metab Dispos. (2002) 30:240–6. doi: 10.1124/dmd.30.3.240
-
30. Avvakumov GV, Cherkasov A, Muller YA, Hammond GL. Structural analyses of sex hormone-binding globulin reveal novel ligands and function. Mol Cell Endocrinol. (2010) 316:13–23. doi: 10.1016/j.mce.2009.09.005
-
31. Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. (2010) 316:3–12. doi: 10.1016/j.mce.2009.06.015
-
32. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. (1989) 10:232–74. doi: 10.1210/edrv-10-3-232
-
33. Fortunati N, Catalano MG, Boccuzzi G, Frairia R. Sex hormone-binding globulin (SHBG), estradiol and breast cancer. Mol Cell Endocrinol. (2010) 316:86–92. doi: 10.1016/j.mce.2009.09.012
-
34. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. (2016) 230:R13–25. doi: 10.1530/JOE-16-0070
-
35. De Moor P, Joossens JV. An inverse relation between body weight and the activity of the steroid binding -globulin in human plasma. Steroidologia. (1970) 1:129–36.
-
36. Javitt NB. Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. FASEB J. (1990) 4:161–8. doi: 10.1096/fasebj.4.2.2153592
-
37. Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharideinduced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. (2007) 117:3979–87. doi: 10.1172/JCI32249
-
38. Selva DM, Hammond GL. Peroxisome-proliferator receptor γ represses hepatic sex hormone-binding globulin expression. Endocrinology. (2009) 150:2183–9. doi: 10.1210/en.2008-1289
-
14. Charni M, Molchadsky A, Goldstein I, Solomon H, Tal P, Goldfinger N, et al. Novel p53 target genes secreted by the liver are involved in non-cell-autonomous regulation. Cell Death Differ. (2016) 23:509–20. doi: 10.1038/cdd.2015.119
-
40. Vandewalle J, Luypaert A, De Bosscher K, Libert C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab. (2018) 29:42–54. doi: 10.1016/j.tem.2017.10.010
-
42. Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. (2005) 34:293–313. doi: 10.1016/j.ecl.2005.01.002
-
43. Snijdewint FG, Kapsenberg ML, Wauben-Penris PJ, Bos JD. Corticosteroids class-dependently inhibit in vitro Th1- and Th2- type cytokine production. Immunopharmacology. (1995) 29:93–101. doi: 10.1016/0162-3109(94)00048-K
-
44. Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. (2004) 2:1–12. doi: 10.2174/1570161043476483
-
45. Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. (1997) 157:792–6. doi: 10.1001/archinte.1997.00440280120012
-
45. Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. (1997) 157:792–6. doi: 10.1001/archinte.1997.00440280120012
-
Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. (2018) 3:509–17. doi: 10.1016/S2468-1253(18)30077-3
-
D’souza AM, Beaudry JL, Szigiato AA, Trumble SJ, Snook LA, Bonen A, et al. Consumption of a high-fat diet rapidly exacerbates the development of fatty liver disease that occurs with chronically elevated glucocorticoids. Am J Physiol Gastrointest Liver Physiol. (2012) 302:G850–63. doi: 10.1152/ajpgi.00378.2011
-
Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, et al. Hepatic steatosis in Cushing’s syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. (2003) 149:543–8. doi: 10.1530/eje.0.1490543
-
Shen M, Shi H. Sex hormones and their receptors regulate liver energy homeostasis. Int J Endocrinol. (2015) 2015:294278. doi: 10.1155/2015/294278
Liver and Steroid Hormones—Can a Touch of p53 Make a Difference?
https://karobben.github.io/2020/07/07/LearnNotes/Paper_Charni2019/







