Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells (Sparus aurata) in response to dietary fish meal replacement by plant protein sources
Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells (Sparus aurata) in response to dietary fish meal replacement by plant protein sources
Cite: Zevallos, Victor F., et al. “Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells.” Gastroenterology 152.5 (2017): 1100-1113.
General Information
Background
Mice:
(图片转自 havahart.com,版权属于原作者)
Abstract
BG
- Wheat amylase-trypsin inhibitors (ATIs)
- ATIs activation of the toll like receptor 4 (TLR4)
METHODS:
Feed to Mice
RESULTS
CONCLUSIONS: Gluten-containing cereals have by far the highest concentrations of ATIs that activate TLR4. Orally ingested ATIs are largely resistant to proteases and heat, and increase intestinal inflammation by activating gut and mesenteric lymph node myeloid cells.
Introduction
###P1: Plant protein substitution for economy
Shortcomings:
- high carbohydrate levels
- isoflavones
- low methionine levels
- anti-nutritional factors
They can:
- impede protein digestion
- impair immune responses
- intestinal inflammation.
End: Yellow perch is one of the most sensitive species for this change.
P2: Pancreas
- Serine-protease (trypsin, chymotrypsin, elastase and collagenase)
- Glucosidases
- hydrolyze starch into lineal chains
P3: Our purpose
- Protine and Carbohydrate digestion
- histology
M&M
- Chemicals
- Diets, animals and growth experiment
- Post-parndial experiment and sampling
- Preparation of extracts and determination of soluble protein
- Enzyme assays
- Zymograms: characterization of protease fractions and inhibition by PP diets
- Proximal intestine histological analysis
- Statistical analysis
Results
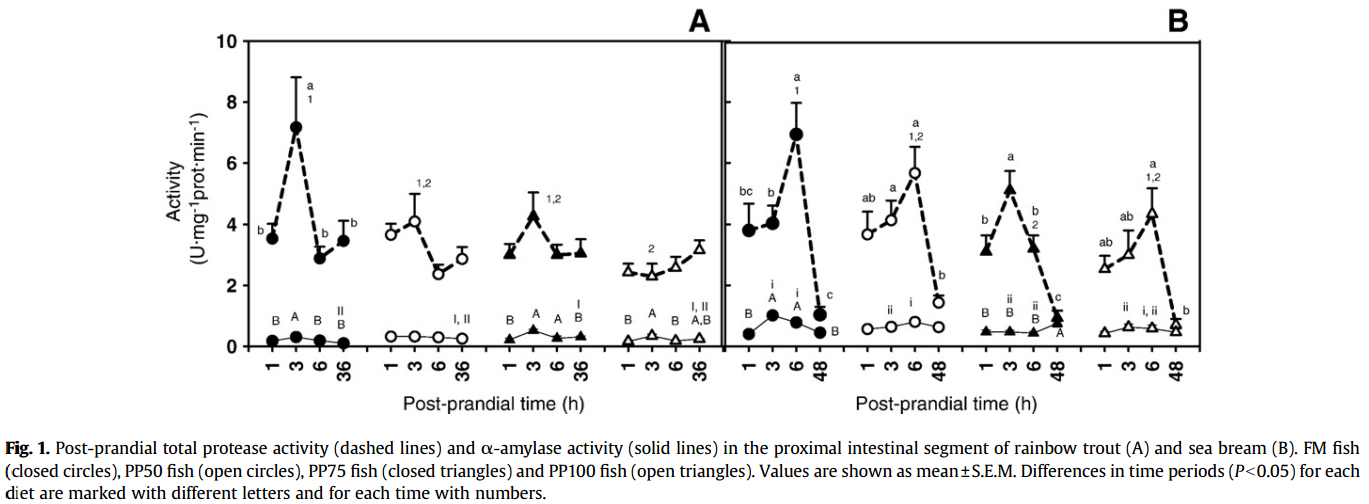
Enzymes activities
Alkaline Protease
- Trout fed diets PP50 and PP75 showed only a slight post-feeding increase in this activity.
- Sea bream fed the FM diet showed a maximum of 6.93±1.03 U protease·mg− 1 protein·min− 1. This value decreased gradually as the percentage of plant protein increased
Amylase
a significant post-prandial increase in this activity was recorded only in PP75 and PP100 fed trout. Replacement of fish meal by plant protein did not induce significant changes in α-amylase activity in either species.
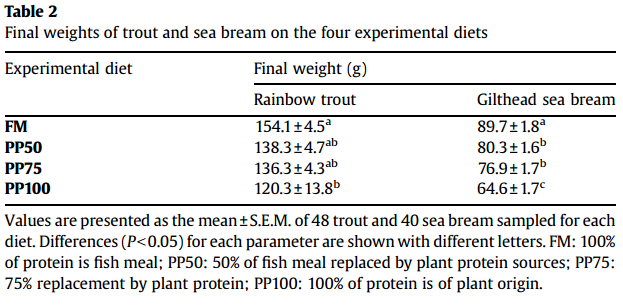
Growth Performance
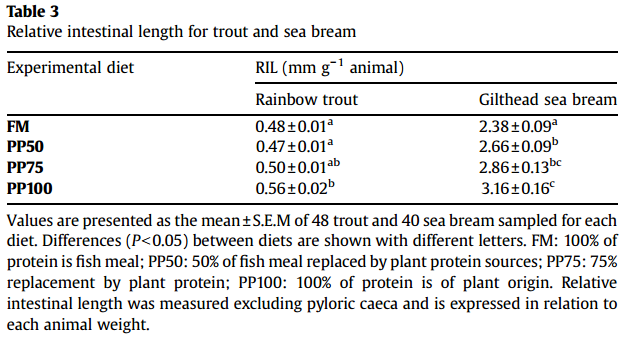
Intestine Growth
Related Notes:
Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells (Sparus aurata) in response to dietary fish meal replacement by plant protein sources
https://karobben.github.io/2020/07/07/LearnNotes/Paper_WheatInhi/








