Principles of Biochemistry 1 |Chiral, Orbital| Class Notes |HarvardX
Unit 1, introduction
Why so much carbon is needed for organism
- It is facility for organism use one molecule for versatility of functions.
- Carbon can build different shape and geometry of molecules:
- linear chain
- branched chain
- cyclical structure
- Structure diversity leads to functional diversity.
Carbon’s versatility
hybrid orbital
 |
|---|
| © courses.lumenlearning.com 3 barbell shape orbital of carbon: 2px, 2py, 2pz |
| There are 2 shells for Carbon. 1s and 2s. In 2s, there is an inner subshell, 2s/2ns, 3 orbital in outer subshell, 2px, 2py, and 2pz |
More for Carbon electron orbital: Chemical Assignment
 |
|---|
| © chemistry-assignment.com |
- Ground state:
6C: $1s2\ |\ 2s2\ |\ 2px1\ |\ 2py1\ |\ 2pz0$ - $sp^3$-Hybridization:
6C: $1s2\ |\ 2s1\ |\ 2px1\ |\ 2py1\ |\ 2pz1$; ($2pz1$ from $2s$) - $sp^2$ Hybridization:
6C: $1s2\ |\ sp^23\ |\ 2px0\ |\ 2py0\ |\ 2pz1$; ($sp^23$ comes from $2s1\ |\ 2px1\ |\ 2py1$) - $sp$-Hybridization:
6C: $1s2\ |\ sp2\ |\ 2px0\ |\ 2py1\ |\ 2pz1$
Ground state
In Ground state, there are two electrons unpaired orbiting in 2p and leaving the 3sd unoccupied orbital 2pz. And this how Covalent Bond formed: one orbital for each atom carrying an unpaired electron.
Hence that, carbon can only from two covalent bonds.
Exited state
The electron in carbon can shift around and so, one electron from 2s promoted into 2spz orbital, to form 4 unpaired electrons.
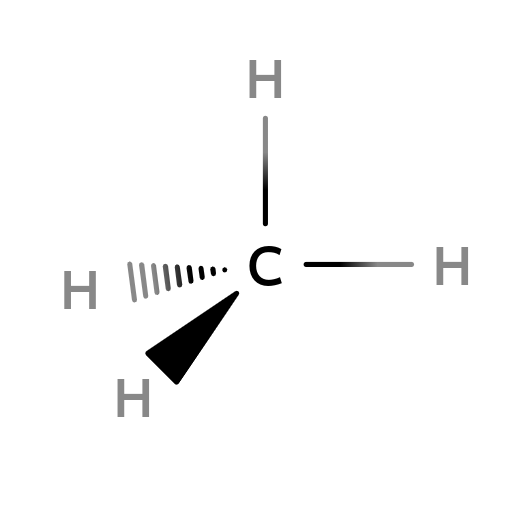
sp3-Hybridization: Mathane (CH4)
$sp^23 = 2s1+2px1+2py1$
4 unpaired electrons are unstable since the orbitals are not identity. So, they have to went through a process name as hybridization to form $sp^3$ orbital. The newly formed $sp^3$ orbital has 25% s character and 75% of p character. For forming a methane, they apart from each other as they can and forming four identical $190.5^{\circ}$ from each other. Which is a tetrahedron.
 |
|---|
| © chemistry-assignment.com |
 |
sp2-Hybridization: ethylene (C2H4)
$sp^2 3= 2s 1 + 2p 2 $
 © chemistry-assignment.com |
|---|
- $sp^2$ has 33% s character and 67% p character and arranged in Trigonal Planar structure. $120^{\circ}$ apart from each other.
- The remaining $2pz$ orbital (Unhybridised 2p-orbital illustrate in the image above.) is orthogonal to the plane containing the $sp2$ orbitals.
~~~~~~~~~~~~~~~~~~~~~~~ |
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ |
|---|---|
 © chemistry-assignment.com |
$\sigma$: sigma
sp-Hybridization: acetylene (C2H2)
$sp2 = 2s+ 2px$
 © chemistry-assignment.com |
|---|
~~~~~~~~~~~~~~~~~~~~~~~ |
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ |
|---|---|
 © chemistry-assignment.com |
The geometry and reactivity of the bonds
| Bond | Length ($A$) | Bond strength ($kcal/mol$) | $\Delta EN$ |
|---|---|---|---|
| $C-H$ | 1.09 | 99 | 0.4 |
| $O-H$ | 0.96 | 110 | 1.4 |
| $N-H$ | 1.02 | 84 | 0.9 |
| $C=O$ | 1.23 | 174 | 1 |
| $C-C$ | 1.54 | 88 | 0 |
| $C=C$ | 1.34 | 147 | 0 |
Shape: $O-H$ is shorter than $C-C$;
Strength: $C-C$ stronger than $C=C$;
Electronegativity: One electron has a stronger pull on the electrons.
Configuration Isomers
Single bond: enantiomer
Molecule containing a chiral center could exist in different configurations.
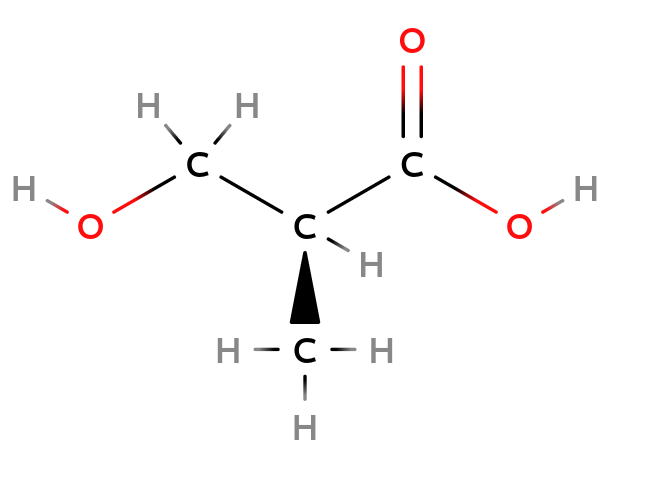 |
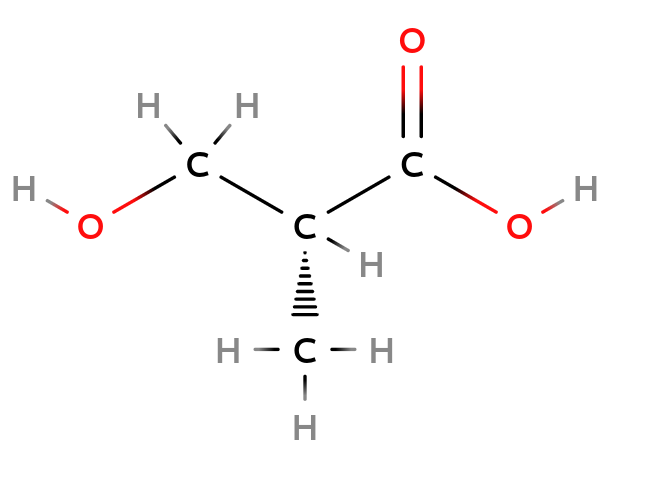 |
Double bond
| Maleix acid | Fumaric acid |
|---|---|
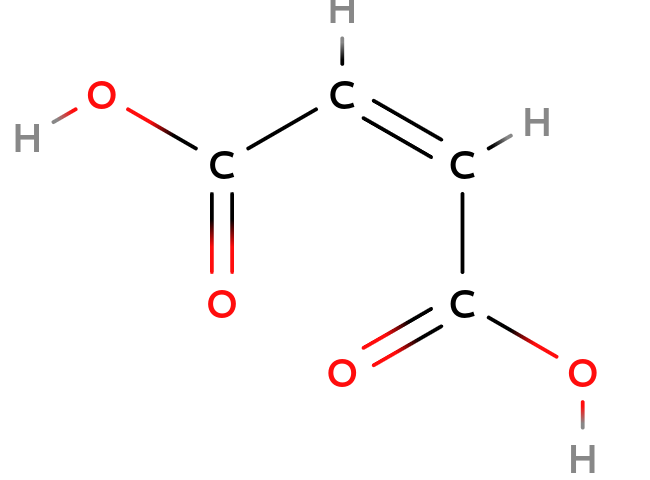 |
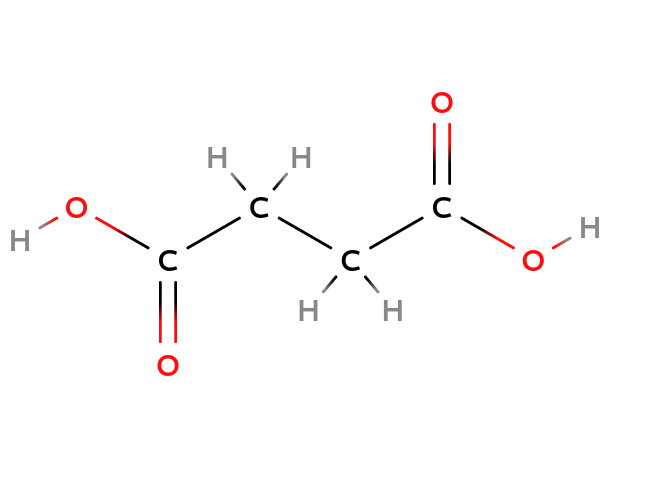 |
| Cis-configuration | Trans-configuration |
As you can see the structure of Maleix acid and Fumaric acid above, they have the same composition but a different structure since the double bond blocked the free rotation of the $C-C$ axis. So, they have the different =configuration=, or =stereoisomers=.
Significant
Same composition with different configuration leads to functional diversity.
exp:
$( R)-Carvone$ and $(S)-Carvone$ bind different neural receptors in your nose.
As a result, $( R)-Carvone$ leads to a fresh spearmint smell, but $(S)-Carvone$ leads to a pungent caraway smell.
| $R-Carvone$ | $S-Carvone$ |
|---|---|
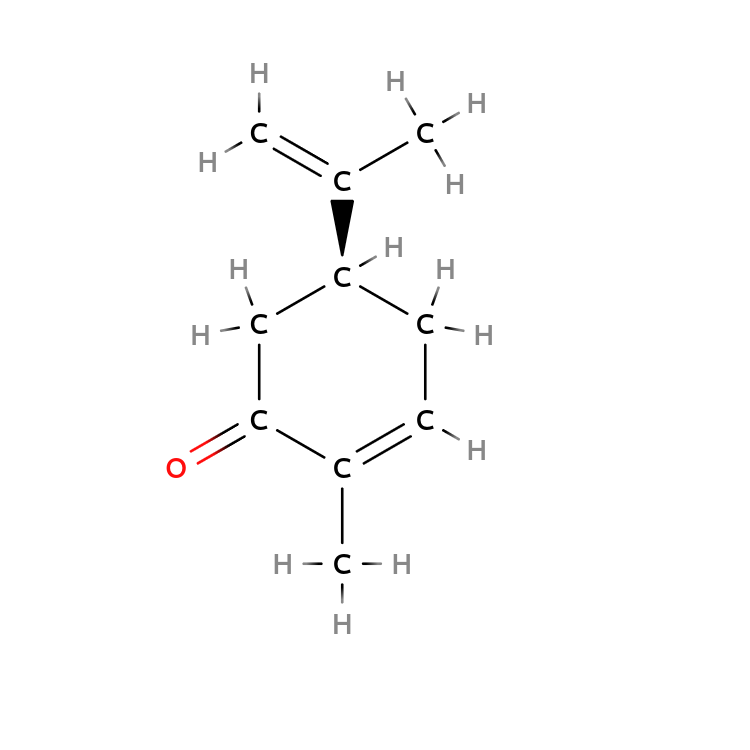 |
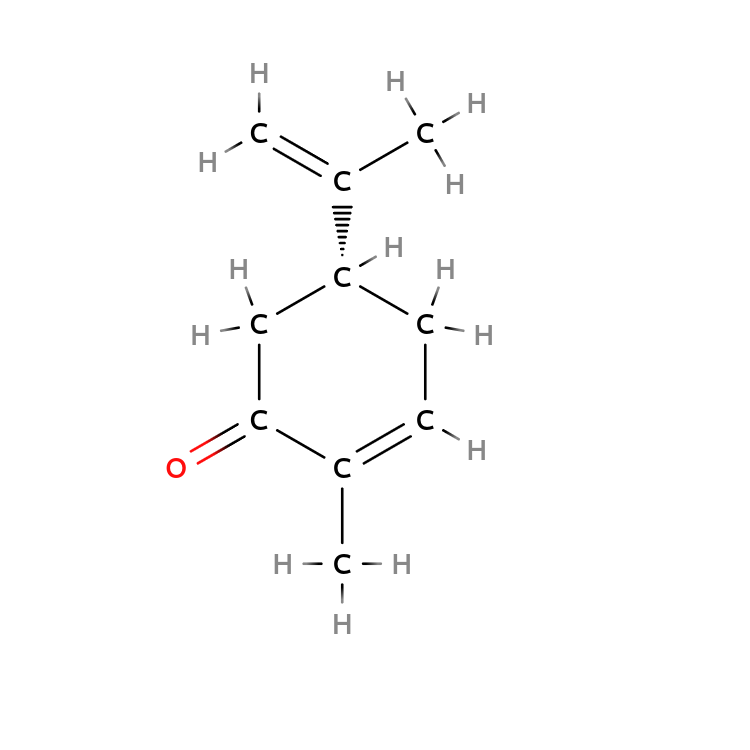 |
Structure Dynamic
Two covalent bonds prohibited rotation. Though single covalent bond made rotation possible and it has infinite number of configurations in theory, majority of them are prefer to stay in a stable structure (low free energy state).
Caffeine from PubChem:
More reading materials about chiral:
libretexts: 5.1: Chiral Molecules
3D models are embedded from molview.org
Principles of Biochemistry 1 |Chiral, Orbital| Class Notes |HarvardX
https://karobben.github.io/2021/03/19/LearnNotes/edx-biochm-1/








