Principles of Biochemistry 11 |Carbohydrates| Class Notes |HarvardX
Introduction
Carbohydrates have structure variety, functional variety, both chemical and physical.
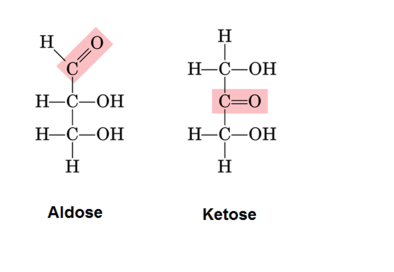
| Aldoses | Ketoses |
|---|---|
| The Chain ends with a carboxyl group | When the carboxyl group was not in the terminal (C2 in nature) |
| -ose | -ulose |
 |
|---|
| © vivadifferences.com |
C3 and C4 carbohydrates are too sample to be found in a complex molecule. They are metabolic intermediates.
C5 pentose has abounded in ribose and deoxyribose form.
C6 hexose was very popular in both structure and energy resources.
Enantiomers
Identify the number of the asymmetry carbon in monosaccharides.
Exp Glyceraldehyde:
- One asymmetric carbon
- Two enantiomers
- Sharing most of the chemical properties
- Affect polarized light
When the polarized light goes through a cell that contains our chiral sugar, the plane of the polarized light will rotate. The rotation direction:
- clockwise: Dextrorotatory; (+)-glyceraldehyde
- counterclockwise: Levorotatory; (-)-glyceraldehyde
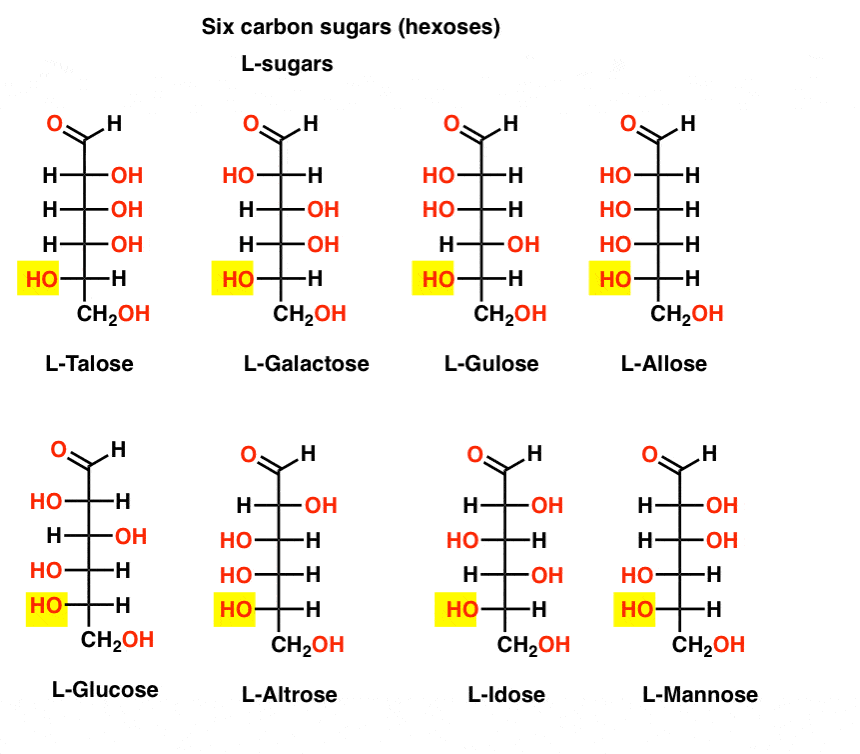
D/L series
 |
|---|
| © masterorganicchemistry.com |
The steps of determing the D/L series:
- Write the developed chemical structure (vertical)
- Place the ketone/aldehyde group at the top of the structure
- Number the carbon started from the top
- Identify the asymmetry carbon.
When the hydroxyl group carried by the bottom-most asymmetry carbon is:
- on the right: D series
- on the left: L series
The D/S series is kind of arbitrary and not really corresponded with enantiomers
Most of the monosaccharides in cells are belong to the D series.
Pentose
 |
|---|
| © Pubchem ID:229 |
Chemical Feature:
Schiff reaction:
- Shiff reagent (pink) + sulfur dioxide -> Discolored
- Adding aldehyde -> recolored (pink)
Hypothesized: the aldehyde is linear: free aldehyde group and it could recolor the Schiff reagent.
Observed: aldose failed to recolor the Schiff reagent
Conclusion: It doesn’t have a free aldehyde group, it’s not linear but cyclic.
Physical Feature:
Theoretically, the D-Glucose has unique physical properties like melting Tm, degree of rotation of polarized light.
But two distinct methods synthesized D-glucose has different physical properties.
| Synthesis | Melting Temperature | Specific Rotation | Form |
|---|---|---|---|
| Methoud 1 | $146^ \circ C$ | $+112^ \circ$ | $\alpha ^ {_ -}D^ {_ -}glucose$ |
| Methoud 2 | $150^ \circ C$ | $+18^ \circ$ | $\beta^ {_ -} D^ {_ -}glucose$ |
Cyclic Structure
Structural analysis a show that hexose and pentose exist in a cyclic structure. The linear structure represents only 0.02% of the molecules of sugar in this solution
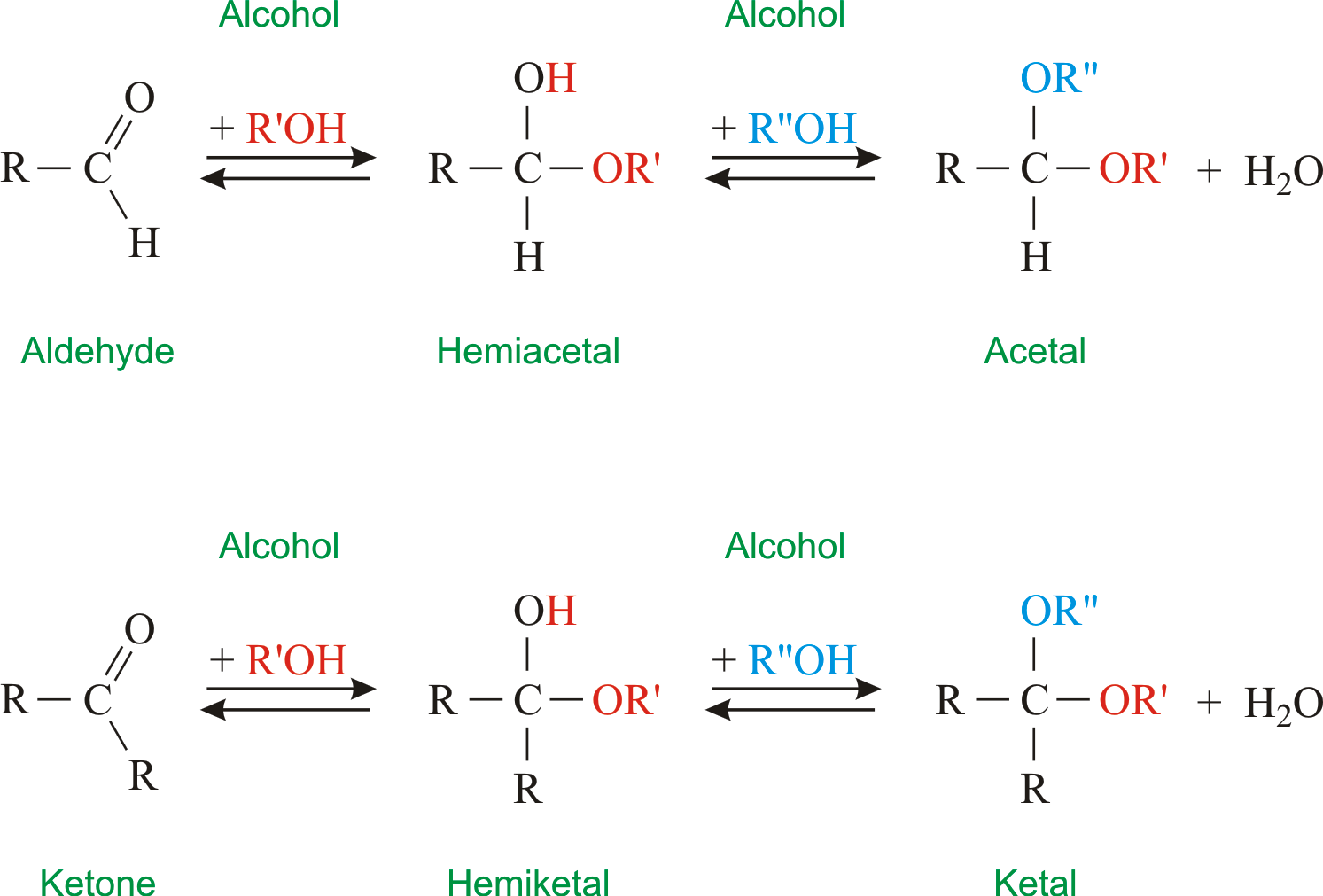
Cyclic Formation
 |
|---|
| © glossary.periodni.com |
$aldehyde + alcohol \to hemiacetal $
$ketone + alcohol \to hemiketal $
D-glucose -> attack the fount or back, to form the alpha or beta cyclic glucose
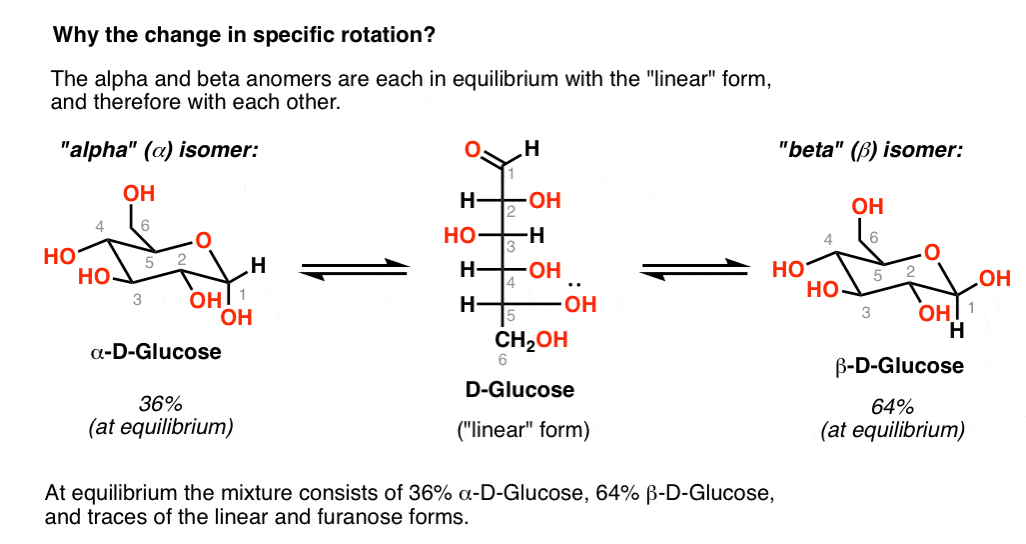 |
|---|
| © masterorganicchemistry.com |
Pryan and Furan
Hexose: Pryan; $\alpha^ {_ -}D^ {_ -}glucopyranose$
Ketose: Furan; $\beta^ {_ -}D^ {_ -}ribofuranose$
Significance
D-Glucose:
- A much stable form: $\alpha^ {_ -}D^ {_ -}glucopyranose$
- A much unstable form: $\alpha^ {_ -}D^ {_ -}glucofuranose$
Conformations
Chair and Boat conformations!
Saccharides poly-formation
| $\alpha^ {_ -}D^ {_ -}Glucose + \beta^ {_ -}D^ {_ -}Glucose$ | $\alpha^ {_ -}D^ {_ -}Glucose + \alpha^ {_ -}D^ {_ -}Glucose$ |
|---|---|
| Maltose: $1 \to 4 glycosidic linkage$ | Trehalose: $ 1 \to 1 glycosidic linkage$ |
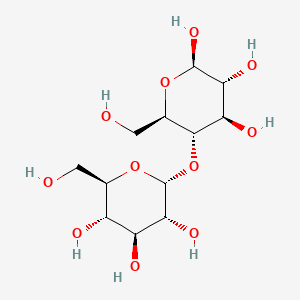 |
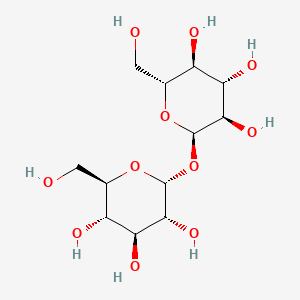 |
| © Pubchem ID:6255 | © Pubchem ID:7427 |
| $\beta^ {_ -}D^ {_ -}Galactose + \beta^ {_ -}D^ {_ -}Glucose$ | $\alpha^ {_ -}D^ {_ -}Glucose + \beta^ {_ -}D^ {_ -}Fructose$ |
|---|---|
| Lactose: $1 \to 4 glycosidic linkage$ | Sucrose: $1 \to 2 glycosidic linkage$ |
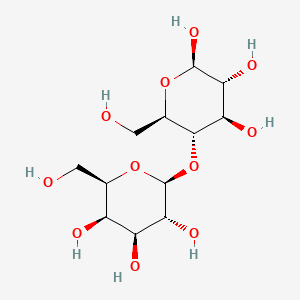 |
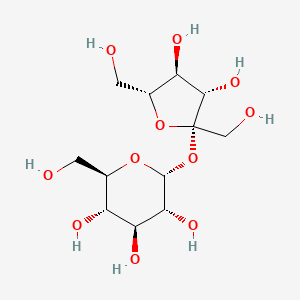 |
| © Pubchem ID:6134 | © Pubchem ID:5988 |
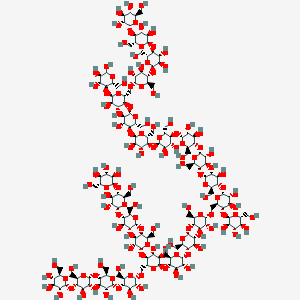
Glycogen
 |
|---|
| © Pubchem ID:146037391 |
Basic Structure
A polymer of $\alpha ^{_ -}D ^{_ -} Glucose$
- Main Stream: reducing end: $1^{_ -} 4\ glycosidic linkage$
- Branch Streams: non-reducing ends: $1^{_ -} 6\ glycosidic linkage$
Synthesis
Initiation
Glycogenin-Try194 + UDP-glucose
CC-glycogen synthase (complex)
glycogenin will catalyze the next three units to form the primer.
Then, the glycogen synthase would able to elongate the chain.
Elongation
Branches ~ every 10 units
up to 55,000 glucose units
~ 2,100 nonreducing ends
Principles of Biochemistry 11 |Carbohydrates| Class Notes |HarvardX
https://karobben.github.io/2021/04/15/LearnNotes/edx-biochm-11/









