3 Extracellular Matrix Adhesion|Advanced Cell Biology|Tulane
Cell-Cell and Cell–Extracellular Matrix Adhesion
In introduction: Cells are actually living in an extracellular material which does not also supply an environment, but also signal contact the cell.
cell-adhesion molecules (CAMs)
Major types of CAMs
- cadherins
- immunoglobulin (Ig) superfamily
- integrins
- selectins
Fibroblast; Tropoelastin; Ground Substance; Tropocollagen
EMC Components
- Fibrous Proteins
- Collagens
- Elastins
- Keratins
- Hydrated Gel (highly sulfide, highly charged carbohydrate molecules)
- Hyaluronic acid
- Proteoglycans
- Multiadhesive Matrix Proteins (two binding sites: matrix components and cell-surface)
- Fibronectin (One site to the binding matrix, another side bonds to Cells.)
- Laminin
- Others
- Cells
- Fibroblasts and mast cells in loose connective tissue
- Epithelial and endothelial cells in basal lamina
- Specialized cells make specialized ECM (chondrocytes/cartilage; osteoblasts/bone; keratinocytes/skin)
Ps: All ECMs are composed of hydrate gel either Hyaluronic acid/Proteoglycans, which are highly sulfate and Carbohydrates to create a highly charged environment and attract lots of water. It could also be attached by proteins.
Functions of the extracellular matrix
- Scaffold for cells
- A barrier to migration (infection, metastasis)
- Reservoir for growth factors
- Signals to the cell interior during morphogenesis wound healing, and maintenance of the differentiated state
Collagen
| © Molview; PDBID=1bkv; Collagen |
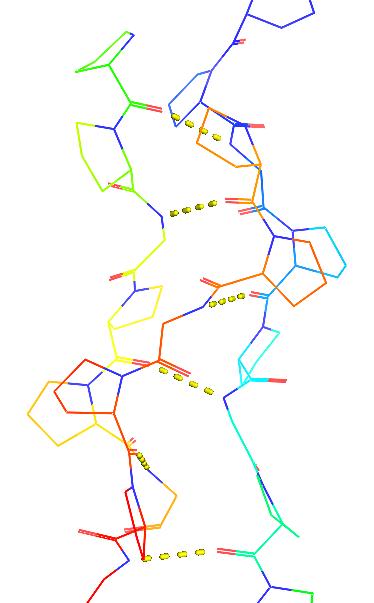 |
Structure
- Most abundant
- Gly-X-Y
- X: mostly was Proline
- Y: mostly was hydrophilic amino acid.
- Type I is called Tropocollagen
- long halve life
Glycines are in the center of the 3 single right-handed α-helix strings coiled fiber.
Structure disruption: Ala replacing Gly causing the deformation of the collagen and cause the “brittle bone”.
Biosynthesis
Collagen was synthesis in the cell but had additional post-translation modification outside of the cell.
- In cell
- Rough ER
- Rough ER translation
- Signal peptide cleavage
- Hydroxylation of selected prolyl and lysyl residues
- addition of N-linked oligosaccharides
- Golgi
- Glycosylation of hydroxylysine residues
- Chain alignment, formation of disulfide bonds
- Formation of triple helix procollagen
- Cytoplasm
- Transport vesicle
- Exocytosis
- Rough ER
It keeps the collagen aggregation outside of the cell
- Out of cell
- Removal of N- and C-terminal pro-sequences
- Lateral covalent cross-linking of tropocollagens
- aggregation of fibrils
Structure of Collagen Genes
A lot of post-translation splicing and modification leads to a lot of genetic diseases.
Gly-X-Y pattern
Whole fasta file: Collagen alpha-1(VII) chain
1260 bp ~ 2783 bp
GEMGLRGQVGPPGDPGLPGRTGAPGPQGPPGSATAKGERGFPGADGRPGSPGRAGNPGTPG
APGLKGSPGLPGPRGDPGERGPRGPKGEPGAPGQVIGGEGPGLPGRKGDPGPSGPPGPRG
PLGDPGPRGPPGLPGTAMKGDKGDRGERGPPGPGEGGIAPGEPGLPGLPGSPGPQGPVGP
PGKKGEKGDSEDGAPGLPGQPGSPGEQGPRGPPGAIGPKGDRGFPGPLGEAGEKGERGPP
GPAGSRGLPGVAGRPGAKGPEGPPGPTGRQGEKGEPGRPGDPAVVGPAVAGPKGEKGDVG
PAGPRGATGVQGERGPPGLVLPGDPGPKGDPGDRGPIGLTGRAGPPGDSGPPGEKGDPGR
PGPPGPVGPRGRDGEVGEKGDEGPPGDPGLPGKAGERGLRGAPGVRGPVGEKGDQGDPGE
DGRNGSPGSSGPKGDRGEPGPPGPPGRLVDTGPGAREKGEPGDRGQEGPRGPKGDPGLPG
APGERGIEGFRGPPGPQGDPGVRGPAGEKGDRGPPGLDGRSGLDGKPGAAGPSGPNGAAG
KAGDPGRDGLPGLRGEQGLPGPSGPPGLPGKPGEDGKPGLNGKNGEPGDPGEDGRKGEKG
DSGASGREGRDGPKGERGAPGILGPQGPPGLPGPVGPPGQGFPGVPGGTGPKGDRGETGS
KGEQGLPGERGLRGEPGSVPNVDRLLETAGIKASALREIVETWDESSGSFLPVPERRRGP
KGDSGEQGPPGKEGPIGFPGERGLKGDRGDPGPQGPPGLALGERGPPGPSGLAGEPGKPG
IPGLPGRAGGVGEAGRPGERGERGEKGERGEQGRDGPPGLPGTPGPPGPPGPKVSVDEPG
PGLSGEQGPPGLKGAKGEPGSNGDQGPKGDRGVPGIKGDRGEPGPRGQDGNPGLPGERGM
AGPEGKPGLQGPRGPPGPVGGHGDPGPPGAPGLAGPAGPQGPSGLKGEPGETGPPGRGLT
GPTGAVGLPGPPGPSGLVGPQGSPGLPGQVGETGKPGAPGRDGASGKDGDRGSPGVPGSP
GLPGPVGPKGEPGPTGAPGQAVVGLPGAKGEKGAPGGLAGDLVGEPGAKGDRGLPGPRGE
KGEAGRAGEPGDPGEDGQKGAPGPKGFKGDPGVGVPGSPGPPGPPGVKGDLGLPGLPGAP
GVVGFPGQTGPRGEMGQPGPSGERGLAGPPGREGIPGPLGPPGPPGSVGPPGASGLKGDK
GDPGVGLPGPRGERGEPGIRGEDGRPGQEGPRGLTGPPGSRGERGEKGDVGSAGLKGDKG
DSAVILGPPGPRGAKGDMGERGPRGLDGDKGPRGDNGDPGDKGSKGEPGDKGSAGLPGLR
GLLGPQGQPGAAGIPGDPGSPGKDGVPGIRGEKGDVGFMGPRGLKGERGVKGACGLDGEK
GDKGEAGPPGRPGLAGHKGEMGEPGVPGQSGAPGKEGLIGPKGDRGFDGQPGPKGDQGEK
GERGTPGIGGFPGPSGNDGSAGPPGPPGSVGPRGPEGLQGQKGERGPPGERVVGAPGVPG
APGERGEQGRPGPAGPRGEKGEA
GEMGLRGQVGPPGDPGLPGRTGAPGPQGPPGSATAKGERGFPGADGRPGSPGRAGNPGTPG APGLKGSPGLPGPRGDPGERGPRGPKGEPGAPGQVIGGEGPGLPGRKGDPGPSGPPGPRG PLGDPGPRGPPGLPGTAMKGDKGDRGERGPPGPGEGGIAPGEPGLPGLPGSPGPQGPVGP PGKKGEKGDSEDGAPGLPGQPGSPGEQGPRGPPGAIGPKGDRGFPGPLGEAGEKGERGPP GPAGSRGLPGVAGRPGAKGPEGPPGPTGRQGEKGEPGRPGDPAVVGPAVAGPKGEKGDVG PAGPRGATGVQGERGPPGLVLPGDPGPKGDPGDRGPIGLTGRAGPPGDSGPPGEKGDPGR PGPPGPVGPRGRDGEVGEKGDEGPPGDPGLPGKAGERGLRGAPGVRGPVGEKGDQGDPGE DGRNGSPGSSGPKGDRGEPGPPGPPGRLVDTGPGAREKGEPGDRGQEGPRGPKGDPGLPG APGERGIEGFRGPPGPQGDPGVRGPAGEKGDRGPPGLDGRSGLDGKPGAAGPSGPNGAAG KAGDPGRDGLPGLRGEQGLPGPSGPPGLPGKPGEDGKPGLNGKNGEPGDPGEDGRKGEKG DSGASGREGRDGPKGERGAPGILGPQGPPGLPGPVGPPGQGFPGVPGGTGPKGDRGETGS KGEQGLPGERGLRGEPGSVPNVDRLLETAGIKASALREIVETWDESSGSFLPVPERRRGP KGDSGEQGPPGKEGPIGFPGERGLKGDRGDPGPQGPPGLALGERGPPGPSGLAGEPGKPG IPGLPGRAGGVGEAGRPGERGERGEKGERGEQGRDGPPGLPGTPGPPGPPGPKVSVDEPG PGLSGEQGPPGLKGAKGEPGSNGDQGPKGDRGVPGIKGDRGEPGPRGQDGNPGLPGERGM AGPEGKPGLQGPRGPPGPVGGHGDPGPPGAPGLAGPAGPQGPSGLKGEPGETGPPGRGLT GPTGAVGLPGPPGPSGLVGPQGSPGLPGQVGETGKPGAPGRDGASGKDGDRGSPGVPGSP GLPGPVGPKGEPGPTGAPGQAVVGLPGAKGEKGAPGGLAGDLVGEPGAKGDRGLPGPRGE KGEAGRAGEPGDPGEDGQKGAPGPKGFKGDPGVGVPGSPGPPGPPGVKGDLGLPGLPGAP GVVGFPGQTGPRGEMGQPGPSGERGLAGPPGREGIPGPLGPPGPPGSVGPPGASGLKGDK GDPGVGLPGPRGERGEPGIRGEDGRPGQEGPRGLTGPPGSRGERGEKGDVGSAGLKGDKG DSAVILGPPGPRGAKGDMGERGPRGLDGDKGPRGDNGDPGDKGSKGEPGDKGSAGLPGLR GLLGPQGQPGAAGIPGDPGSPGKDGVPGIRGEKGDVGFMGPRGLKGERGVKGACGLDGEK GDKGEAGPPGRPGLAGHKGEMGEPGVPGQSGAPGKEGLIGPKGDRGFDGQPGPKGDQGEK GERGTPGIGGFPGPSGNDGSAGPPGPPGSVGPRGPEGLQGQKGERGPPGERVVGAPGVPG APGERGEQGRPGPAGPRGEKGEA
|
|
Collagen classes and types:
- Fibrill-forming
- Type 1: Most abundant connective tissue, which is long rode triple collagen. (fiber-forming)
- Type 2: Cartilage and vitreous humor. (Most fiber forming collagen)
- Type 4: (Basal lamina) Chicken Wire shape, to reinforce the structure.
- Network-forming
- Type 7: Anchoring fibrill which holds the dermis and epidermis.
- Anchoring filament
- Type 8: Attachments of basal laminae to underlying connective tissue. (Butterfly Children: lack a particular collagen, gentle touch in the skin could cause blister)
Elastin
 |
|---|
| © Y.K. Lahir |
- A highly hydrophobic protein rich in glycine and proline. |
 © Valentin Hagel, et al |
Keratin
The major fibrous protein of hair, nails, and the enamal of teeth. These fibers are embedded in a ground substance that consistance of keratohyaline and proteoglycan. |
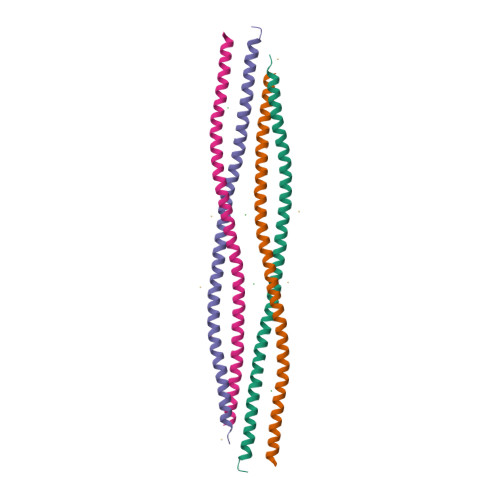 © PDB id=6EC0 |
Glycosaminoglycans
core introduction: NCBI Books
- Glycosaminoglycans (GAGS) are unbranched polysaccharides composed of repeating disaccharides units. (mostly: uronic acid and amino sugar)
- Glycosaminoglycans are classifide primarily according to the amino sugar they contain.
- Because of their highly charge, GAGs are osmotically active and imbibe large quantities of water.
- GAGs form the porous hydrated gels that fill most of the extracellular space. Collagen and elastic fibers comprise the reinforcing fiber embedded in this “ground substance”.
uronic acid and sulfide compounts contributed the negative charge of the Glycosaminoglycans
Proteoglycans
 |
|---|
| © Hussein Kaoud |
- Most GAGs (with the exception of the hyaluronic acid) are covalently linked to a protein core.
- These covalent conjugages are called proteoglycans. Proteoglycans are normally given names when the protein core to which the GAG is attached has been cloned and sequenced.
- Formerly, the core proteins of the proteoglycans were thought to have a passive role of carry glycosaminoglycan GAG chains. It appears now, however, that, the core protein so of the some molecules are involved in mediating outside-in signals generated by the binding of ligands.
Aggrecan
Aggrecan is a type of Proteoglycans
 |
|---|
| © Ameera Kamal Khaleel |
Electron micrograph and diagram of the structure of aggrecan, a proteoglycan aggregate found primarily in the specialized connective tissue, cartilage.
PS: sulfate proteoglycan gives lots of charges which makes this structure work like a sponge.
| Proteoglycan | Mol Wt of core protein | Type of GAG chain | Number of GAG Chain | Location | function |
|---|---|---|---|---|---|
| Aggrecan | 210,000 | Chondroitin and Keratan sulfate | ~130 | Cartilage | Mechanical support; forms large aggregates with hyaluronic acid, binds TGF-β |
| Perlecan | 600,000 | Heparan sulfate | 2-15 | Basal lamina | Structural and filtering function in basal lamina |
| Syndecan-1,2,3 and 4 | 32,000 | Chindroitin sulfate + heparan uslfate | 1-3 | Fibroblast, epithelial cell surface | Cell adhesion, binding FGF; Signal Transduction |
Multiadhesive Matrix Proteins
 |
|---|
| © Veli-Jukka Uitto |
- Fibronection: The most abundant of the ECM adhesion protein; has binding site for type I collagen, fibrin, heparan sulfate proteoglycans, and membrane-intercolated receptors.
- Laminin: AN important component of the basal lamina synthesized by epithelial and endothelial cells. Laminin is the first ECM adhesion protein to appear during embryonic development and appears to be very important in the development of the nervous system.
- Thrombospondin: An important component in platelet aggregation. This cell is also involved in endothelial cell proliferation.
Fibronectin
Fibronectin consist of two nearly identical chains joined by two sulfide bonds near their carboxyl ends.
RGD domain was recognized by fibronectin receptors
Laminin-1 is synthesized by epithelial and endothelial cells and is the principal multi-adhesive matrix protein of the basal lamina. At least 8 different genes code for laminin-like proteins.
Transmembrane Protein
| Family | Ligands recognized | stable cell junctions |
|---|---|---|
| Selectins | Carbohydrates | No |
| Integrins | ECM Members of lg superfamily |
Focal adhesions and hemidesmosomes No |
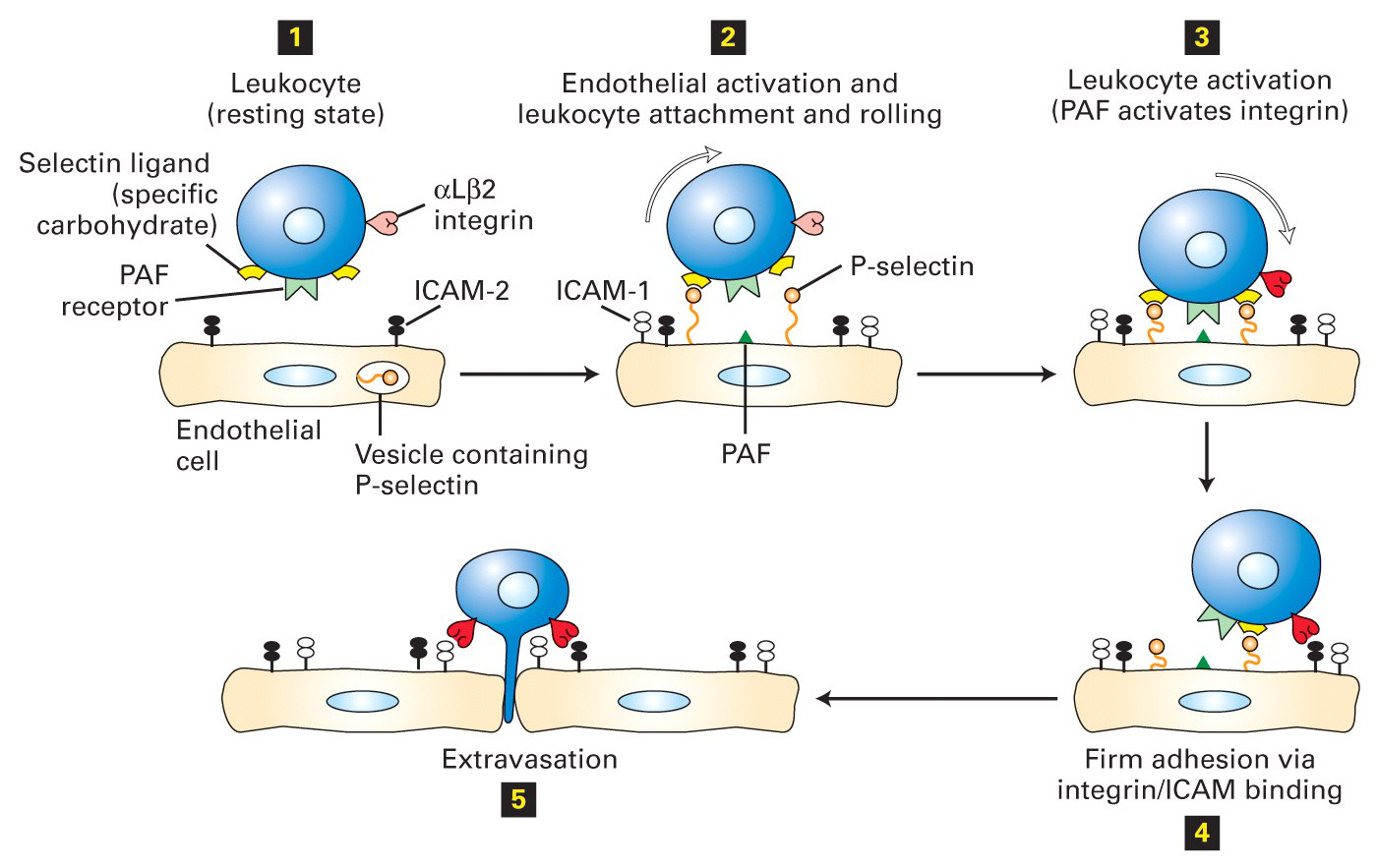 |
|---|
| [Source: Don’t know = =] |
Disruption of this process decreases inflammation
- A humanized monoclonal antibody, Natalizumab, which binds to the α4 integrin subunit, disrupts the ability of leukocytes to move from the bloodstream to the tissues.
- This antibody has been shown to be effective in reducing the severity of symptoms in Multiple Sclerosis, most of which can be attributed to inflammation.
- A low molecular weight agonist (activator) of the αMβ2 integrin also prevents extravasation of the leukocytes and decreases inflammation. This drug is not yet in clinical trials. Modulating integrin action decreases inflammation
Integrins
- Integrins are obligate heterodimers containing two distinct chains, called the α and β subunits.
- In mammals, 19 α and 8 β subunits have been characterized.
- There are many types of integrins, and many cells have multiple types on their surface.
Integrin Structure.
- Both the alpha and beta subunits are attached to the cellular plasma membrane through a single transmembrane helix.
- The binding specificity of integrins depends upon the nature of the alpha and beta subunit.
- Most integrins recognized relatively short peptide motifs and in general require an acidic amino acid to be present.
Integrin Function
- Many integrins are expressed on the surface of cells in an inactive state, where they do not bind ligands and do not signal.
- Integrins interact with the elements of the extracellular matrix and mediate various intracellular signals.
- They define cellular shape, mobility, and regulate the cell cycle.
Integrin Signaling
- Integrins communicate biochemical and mechanical signals in a bidirectional manner across the plasma membrane.
- Intracellular signals switch integrins into a ligand-competent state as a result of conformational changes in the extracellular domain.
- Binding of extracellular ligands induces, in turn, structural changes that convey distinct signals to the cell interior.
cell signaling
- Structural adaptors (e.g. talin, tensin,) link integrins directly to the cytoskeleton
- Scaffolding adaptors (e.g. paxillin, kindlin) forms bridges between focal adhesion proteins.
- Catalytic adaptors (e.g. focal adhesion kinase, integrin-linked kinase, Src) propagate signal transduction from adhesion sites. Phosphorylation state of cytoplasmic tail residues modulate the competition between adaptors for binding and hence the subsequent cytoskeletal interactions of integrins and response.
Interactions between beta integrins and signaling proteins
FAK, focal adhesion kinase
p130Cas, an adaptor that promotes protein–protein interactions, leading to the formation of multiprotein complexes
Crk, a family of signal transducing adaptor proteins that contain SRC homology domains and play a role in cytoskeletal reorganization.
Abl, cytoplasmic and nuclear protein tyrosine kinase that has been implicated in processes of cell differentiation, cell division, cell adhesion, and stress response.
Be able to name the proteins that integrins use for signal transduction.
Integrins activate intracellular signaling pathways
- Which signaling pathway is initiated by integrin activation is based on the biological context, as well as the ligands bound (matrix components, growth factors).
- Depending on the combination of these factors, a variety of short-term and long-term responses may result.
- Substrate stiffness has also been shown to affect the type of adhesion structure formed following integrin activation.
Integrin signals intersect growth-
factor pathways at multiple points. PAK proteins are critical effectors that link Rho GTPases to cytoskeleton reorganization and nuclear signaling.
They consist of a family of serine/threonine p21- activating kinases, serve as targets for the small GTP binding proteins Cdc42 and RAC and have been implicated in a wide range of biological activities.
Key Points to Remember
- Components of the extracellular matrix
- Collagen structure (types I, II, IV and VII) and the importance of glycine in the triple helical region.
- Proteoglycan structures (especially Aggrecan)
- Transmembrane proteins that mediate cell adhesion and their specificity
- Steps in extravasation of leukocytes
- Integrin structure (activated and unactivated) and specificity
- Intracellular proteins involved with integrin signaling
3 Extracellular Matrix Adhesion|Advanced Cell Biology|Tulane
https://karobben.github.io/2021/09/02/LearnNotes/tulane-cellbio-3/








