Principles of Biochemistry 9 |Lipids Structure And Membrane Assembly| Class Notes |HarvardX
Lipid Acid
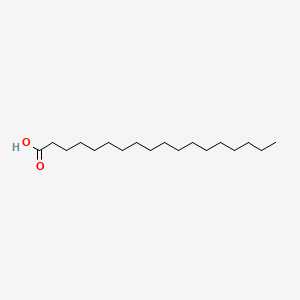
Fatty Acid
Structure: Carboxyl head + Hydrocarbon tail.
 |
|---|
| © pubchem; ID:5281 |
- The length of the tail is variable
- The tail could be saturated or unsaturated (C=C double bond)
- Interacted with each other through **Van der Waals interaction.
- With a longer tail and more unsaturated bond, the fatty acid has more interactions and could pack together more tightly like animal fat.
- Most of them have an even number of the carbon
Incorporated into lipids
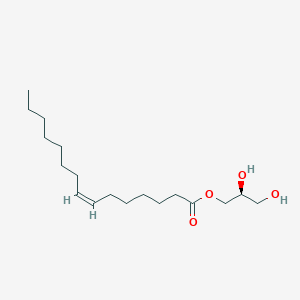
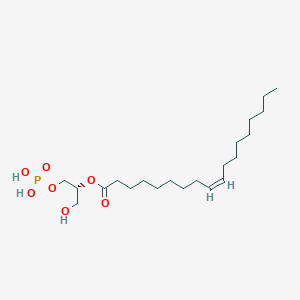
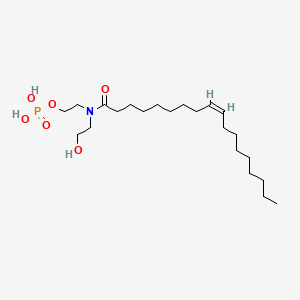
The tail of the fatty acid could link with different molecules to form different linkages.
- ester linkages
The hydroxyl group in glycerol reacts with carboxyl group in fatty acid to form ester linkages. - Phosphodiester linkage
Phosphate group with hydroxyl group on glycerol or Sphingosine - amide bond
Carboxyl group links to the amino group of a Sphingosine forming an amide bond
 |
 |
 |
|---|---|---|
| © pubchem; ID:70698413 | © pubchem; ID:52929749 | © pubchem; ID:6437496 |
Triacylglycerol
Three fatty acids form 3 ester linkages with glycerol.
The 3 fatty acids could be either identical or mixed.
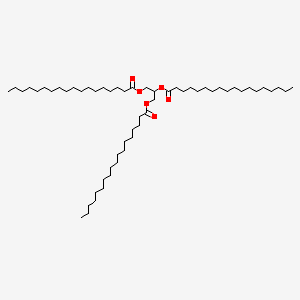 |
 |
|---|---|
| © pubchem; ID:11146 | © pubchem; ID:131762421 |
Structural Lipids
Two major structural lipids:
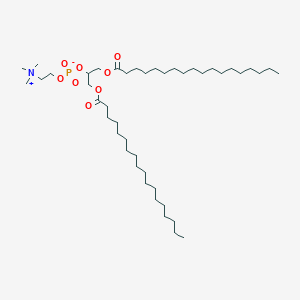
- glycerophospholipids (phosphoglycerides)
- glycerol backbone
- Phosphodiseter linkage that connects the third carbon to a variable
- Glycerol C1: Saturated fatty acid (C-16 ~ C-18)
- Glycerol C2: Unsaturated fatty acid (C-18 ~ C-20)
- Glycerol C3: Phosphate group which connected a hydrophilic head. The simplest head is the phosphate group connected with hydrogen, which is phosphatidic acid
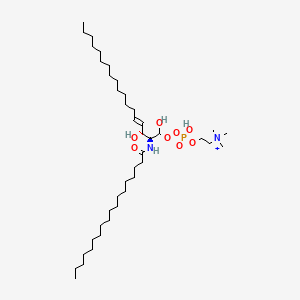
- sphingolipids
- A molecule of sphingosine forms the backbone
- Both sphingosine and fatty acid groups are hydrophobic
- The variable group determined the function of the molecule
- Both the variable group and the fatty acid tails affect the function of the molecule.
 |
 |
|---|---|
| © pubchem; ID:192817 | © pubchem; ID:319423558 |
The shape of fatty acids
| Illustration of the Cylindrical Shape and Conical Shape |
|---|
 |
| © Bruno Maggio |
Some structural lipids, such as phosphatidylcholine have a cylindrical shape, while other lipids, like phosphatidylethanolamine, have a conical shape, because they have a relatively small head group, and carry unsaturated fatty acids.
As a result, placing more conical shape molecules in the inner layer helps the structure of the membrane more tightly.
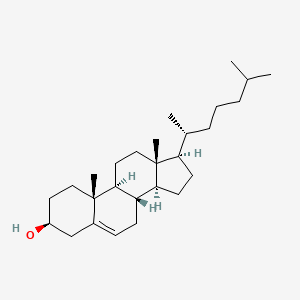
Cholesterol
 |
|---|
| © pubchem; ID:5997 |
Cholesterol has four hydrocarbon rings and is very hydrophobic. It can interact with other structural lipids in the membrane. It limits lipids’ mobility and affects membrane fluidity.
There are more than 1000 types of lipids we can find in an organism. And About 5% of the genes in a cell are for synthesizing lipids.
Energy production of lipids
Triacylglycerol
Most Triacylglycerols are stored in a kind of cell named Adipocyte (Fat cell) in adipose tissue. With the adipocyte, the triacylglycerol was stored as very large lipid droplets which even against the membrane of the adipocyte.
- Immature adipocyte tissue: brown, large lipid droplets.
- Mature liver adipocytes: small and numerous lipid droplets.
The DIFFERENT between Glycogen and triacylglycerol:
-
- The carbon in fatty acids is more reduced. As a result, one gram of the fatty acid has twice the energy as 2 gram glycogen after being completely oxidized.
- Hydrophobicity: The fatty acid was stored much tenser than the glycogen. The glycogen has 2/3 of the mass of water.
- Glycogen is a polymer of glucose units.
Glycogen moves faster than triacylglycerol.
On the other hand, triacylglycerol also contributed to the benefit of insulation and padding.
Lipid Transportation
Challenge 1: Hydrophobic
- Lipid droplets are surrounded by a monolayer of phospholipids with hydrophilic group heads outside.
- lipid-protein: perilipin, which could form a layer on the outer surface of the lipid droplets.
Challenge 2: Coordination
Triacylglycerol degradation (Lipolysis):
- tri -> di: ATGL (Adipose triglyceride lipase);
- di ->Mono: HSL (Hormone-sensitive lipase);
- Mono->two: MGL (Monoacylglycerol lipase);
-
How are these enzymes activated?
- ATGH needs ABHD5 to be recruited at the surface of the lipid droplets.
- HSL and FABP4 complex in free cytoplasm could not be recruited.
- Active: Hormones such as adrenaline and noradrenaline from the catecholamine family.
-
When are they activated?
- Catecholamine (Receptor) -> trimeric G protein.
- trimeric G protein -> adenylyl cyclase
- adenylyl -> ATP -> cyclic AMP
- Cyclic AMP -> PKA
- PKA -(phosphate)-> perilipin and HSL
- Perilipin-P
- active ABHD5 to recruit ATGL
- remodeling the surface of the droplets, increasing the access of the enzyme
- HSL-P: It could be recruited by the surface of the droplets.
Challenge 3: Release of fatty acid
- Free fatty acid + FABP4 (chaperone): through the cytoplasm.
- FA is exposed to the bloodstream and associated with serum albumin to travel into the tissue in need.
- Glycerol was exposed in the cytoplasm and went through the cytomembrane through aquaporin, a channel protein. And then, they were transported into the liver through the bloodstream. (No chaperone, it’s hydrophilic)
Principles of Biochemistry 9 |Lipids Structure And Membrane Assembly| Class Notes |HarvardX
https://karobben.github.io/2021/04/13/LearnNotes/edx-biochm-9/








