Water, thermodynamic|Graduate Biochemistry 1| Tulane
| Co-Author: Haoyang liang |
|---|
Structure of Water
What makes water unique
-
Dipole
-
oxygen is tetrahedral (sp3 hybridization)
-
4 orbitals
-
4 pairs of electrons
- 2 of them bind to protons
- 2 of them are just orbitals
-
orbital is not completely random
- Electrons spend more time around positively charged protons.
- which cause the water dipole
-
In a right distance and right angle, they’ll form a hydrogen bond
-
In the water, there are lots of polar, dipole interactions which not form hydrogen bonds.
-
Hydrogen bond was much dominant in the water interaction with other biomolecules.
-
-
Small Size
- small size and so, you have extrema abundant of a polar group.
Hydrogen bond
How it formed:
- Require hydrogen donor (δ+) and hydrogen acceptor (δ-) groups
- A hydrogen acceptor has a free electron pair
- Hydrogen is shared between the groups
- Separation and geometry are conserved
Common Atoms you know- Oxygen has 2 bonds and 2 free electron pairs (only accept)
- Nitrogen often has three bonds and 1 free electron pair and sometimes 4 bonds and no free electron pairs (accept other protons)
- Carbon has four bonds and NO free electron pairs and does not form hydrogen bonds
Double bonds make the atoms in a co-plane.
Other things about water
The number of hydrogen bonds
- Liquid water: ~3 hydrogen bonds/molecule.
- Ice: 4 Hydrogen bonds/molecule.
Quantity:
- The molar concentration of water is ~55M.
- The concentration of Hydrogen bonding groups in water is 220 M.
- Which makes water is extremely polar
- This character drives the most unique properties of the water
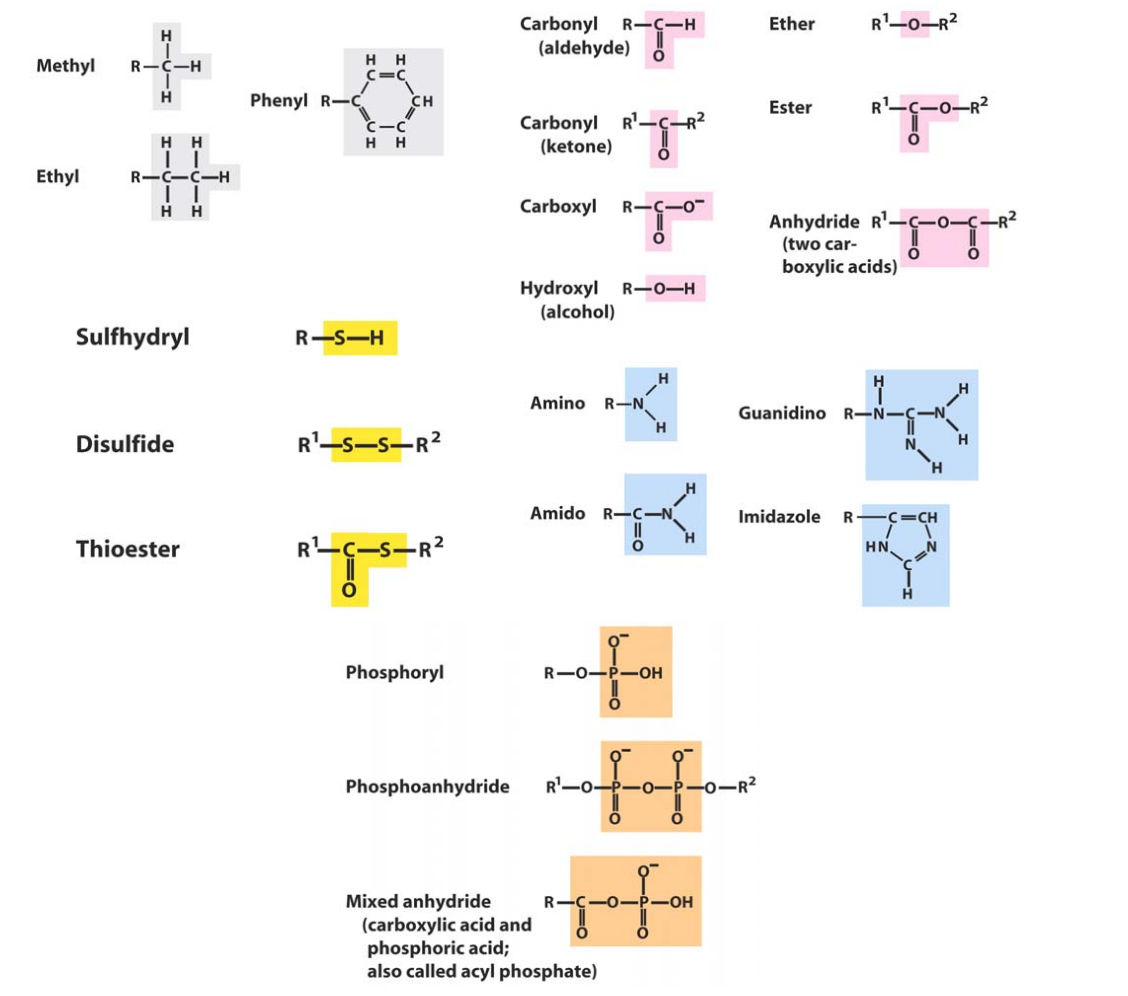
Common Chmeical Group
- Remember them all in slides - -
- Thinking about hydrogen bonding.
DNA pairs
 |
|---|
| © ATbio |
Ionic Interaction
 |
|---|
| © wps.prenhall.com |
All polar molecular has dipoles. This property makes them can interact with a hydrogen bond.
The water automatically circles the dipole molecules by ion interaction.
- charge-charge (can over distance)
- Dipole-dipole (weak than c-c)
- charge-dipole interactions
- Exp: water circle the charged particles to form a water shield.
- it limited the charge-charge interaction
At the surface of the protein, not surrounded by water, the interaction of molecules here could become much stronger. (Con contain? ion, not shield by water)
- Dielectric constant of the medium (weaker in more polar solvents)
- Ionic strength of the solution (weaker as ion concentration increases)
Hydrophobic Interaction
The measure of the non-favorite interaction of nonpolar molecules
A non-polar molecule or group is “Hydrophobic”
doesn’t solve into water
Being nonpolar, not really interact with water.
- surface tension
- water molecules can’t H bond with nonpolar solutes so they H-bond with each other, forming a “cage”
- this ordering (entropy) is unfavorable
- the hydrophobic effect is proportional to the nonpolar surface area that is exposed to water
Exp:
- a bottle of water, take non-polar (CH4) into the water, happens nonthing
- remove water-water interaction: lost some classic phenomenal
summary of interaction energies
- Covalent
- Ionic
- Hydrophobic
- Thermol dynamic favorable.
- Hydrogen Bond
- Dipole-Dipole
- Induced dipole (Van Der Waals)
Ionization of Water
$H_ 2O \longleftrightarrow H^ + + HO ^ -$
$$
k = {\frac{[H^ +][OH^ -]}{[H_2 O]} } = 1.82 \times 10^ {-16}\ Molar
$$
Since H2O is constant (55.3 M), we can write
$$
K^ * = k[H_2 O] = [H^ +][OH^ -] = 1.01 \times 10^{-14}
$$
which usually rounded into 10-14
$$
pH = -log([H+])
$$
Ionization Equilibria
low Ph -> proton concentration is high in water
high Ph -> low proton concentration
$$
pK_ A = -log(K_ A) = -log(\frac{[H^ +][A]}{[AH]})
$$
$$
pK_ A = -log([H^ +])-log(\frac{[A]}{[AH]})
$$
Henderson-Hasselbach Equation
$$
pK_ A = pH - log(\frac{[A]}{[AH]})
$$
Middle of the curve is pKa: point tow portion equally abounded
Thermodynamic and Energetics
- Spontaneity of chemical reactions
- Chemical Potential, Equilibrium, standard states
- Equilibrium constants & Free Energy
- Coupled reactions
- High energy compounds
- Metabolic pathways - glycolysis
Second Law of Thermodynamic
In biology, we focus in:
- constant pressure, constant temperature
$\Delta H - T \Delta S < 0 $ is the energy we can use
$- \Delta G$ is spontaneous, is a thermodynamically favorable reaction
$aA + bB \longleftrightarrow cC + dD$
$$
\Delta G = \Delta G^ {\circ} + RT\ ln[\frac{C^ c D^ d}{A^a B^b}]
$$
The rate of a chemical reaction is INDEPENDENT of $\Delta G,\ \Delta G^ {\circ}$
$$
\Delta G ^ {\circ} = - RT\ ln[K_ {eq}]
$$
- Enzymes CAN NOT change the equilibrium concentrations in a reaction
- Enzymes can ONLY allow reaction to proceed towards equilibrium
Metabolic pathways
- often operate far from equilibrium
- are steady state pathways
- are irreversible because at least one step has large negative $\Delta G ^ {\circ}$
- The forward pathway is highly favorable (the sum of all the ΔGo is negative)
- Flow through the pathways is from A to J (the sum of all the ΔG is negative)
- Reactions 2,6,7 and 8 are near equilibrium
- Reaction 4 (D→E) is highly unfavorable
- Reactions 1,3, 6 and 9 are highly favorable
- Reactions 1,3 and 9 are far from equilibrium
Water, thermodynamic|Graduate Biochemistry 1| Tulane
https://karobben.github.io/2021/08/25/LearnNotes/tulane-biochem-1/








